Bioanalytical Method Development: Focus on Polar Compounds

Bioanalysis is a subdiscipline of analytical chemistry used in the identification and quantitative measurement of target analytes, such as drugs and their metabolites or biological molecules, in biological fluids or tissues. In the pharmaceutical industry, bioanalysis is carried out to support pharmaceutical applications, for example pharmacokinetic- (PK) or pharmacodynamic (PD) studies.
Rapid growth of bioanalysis using liquid chromatography with tandem mass spectrometry (LC–MS–MS) in pharmaceutical laboratories has been driven by the demand for speed and sensitivity at various stages in drug development, including high throughput screening of drug candidates, rapid data generation at pre-clinical studies to fast analysis of clinical samples.
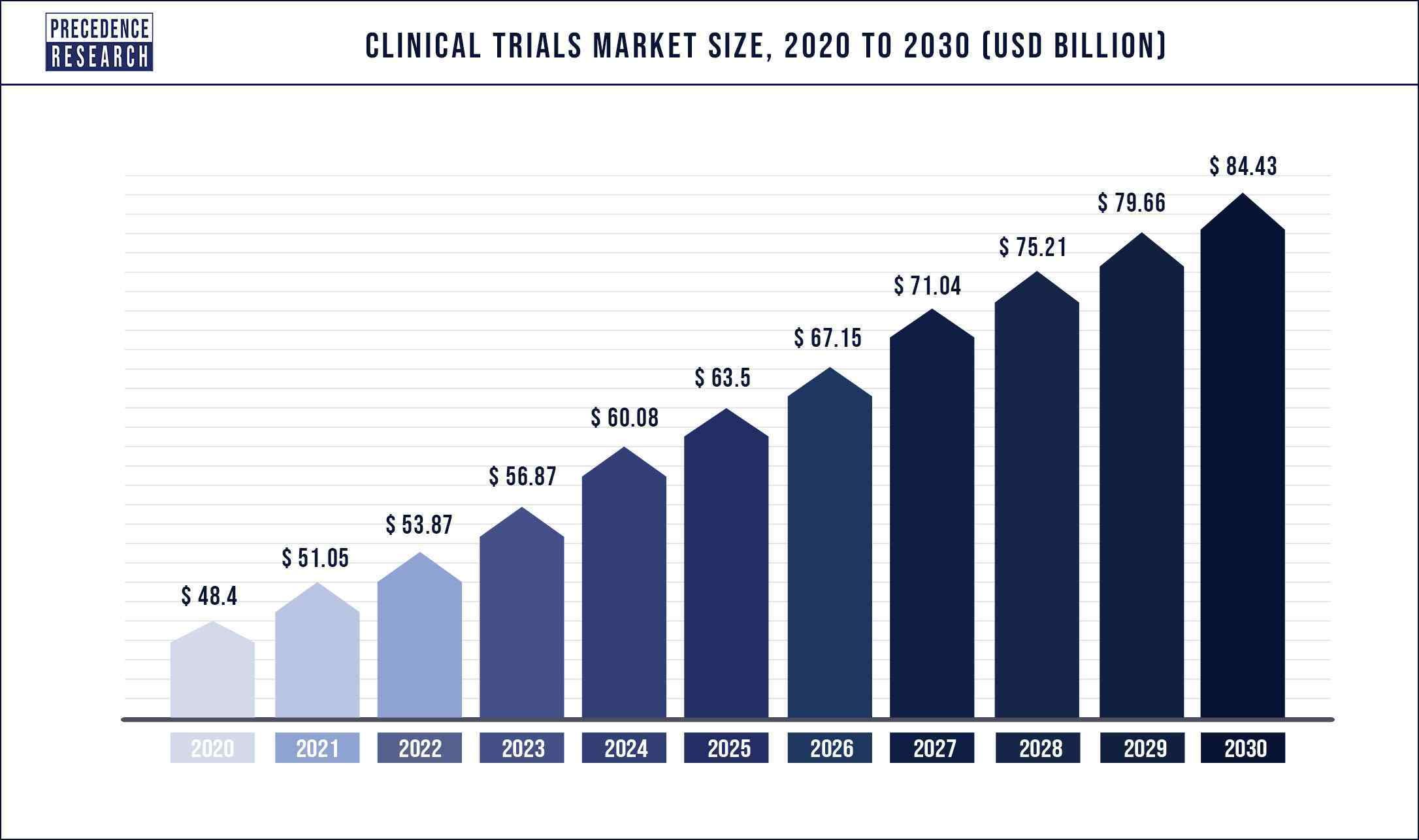
According to a new research report by Precedence Research, the global clinical trials market size was valued at USD $51 billion in 2021 and is predicted to hit USD $84 billion by 2030 with a compound registered annual growth rate (CAGR) of 5.7% during the forecast period 2022 to 2030. Also, the GlobeNewswire reports that the global bioanalytical testing services market size is expected to reach almost USD 8 billion by 2030. Registering a CAGR of 8.6% over the forecast period.
Why quantitative analysis of polar compounds is a challenge:
Liquid chromatography-tandem mass spectrometry is established as the state-of-the-art methodology for quantitative bioanalytical assays in human plasma samples, however development of LC-MS/MS methods for the quantitation of very polar compounds poses the greatest challenge for bioanalytical research scientist.
Polar compounds possess the following characteristics:
-
- Contain a prominent polar functional group in their chemical structure, such as guanidine, nucleoside, amino acid, sugar moieties and phosphonate;
- Highly hydrophilic and immiscible with commonly used organic solvents (e, g., ethyl acetate, diethyl ether and dichloromethane).
- Polar compounds are poorly retained on reversed phase (RP) HPLC columns.
- For bioanalytical LC-MS/MS application, especially when using electrospray ionization (ESI) mode, poor analyte on-column retention may result in detrimental matrix effects that debilitates the robustness of the method, a greater possibility of interference from endogenous molecule present in matrix and poor sensitivity.
Quantitative determination of polar compounds in biological matrixes at BioPharma Services:
Examples of polar drugs quantified in plasma sample by LC-MS/MS in Biopharma
| Method | Compound | Calibration Range | Extraction | Chromatography column | Mobile Phase /Flow rate |
|
1
|
Morphine Morphine glucuronide |
0.10 to 20 ng/mL 1.0 to 200 ng/mL |
Waters Oasis HLB SPE | Thermo Betasil C8 | Gradient elution: 3.5 mM ATFA/Methanol |
|
2
|
Trientine N1-Acetyltriethylenetetramine |
10 to 2000 ng/mL 10 to 2000 ng/mL |
PPT & Oasis HLB SPE | Phenomenex Kinetex C18 | Gradient elution:0.2% Heptafluorobutyric Acid water/Methanol |
|
3
|
Amlodipine Lisinopril |
0.15 to 30 ng/mL 1.5 to 300 ng/mL |
Phenomenex Strata X SPE | Phenomenex Luna Omega Polar C18 | Gradient elution:2.5 mM ATFA water/Methanol |
| 4 | N-Acetyl-D-Alanine | 1.0 to 250 μg/mL | PPT with Acetonitrile | Waters Atlantis Hilic Silica | 10.0 mM ammonium formate with 0.1% acetic acid in water/Acetonitrile |
| 5 | Vancomycin | 1.0 to 250 µg/mL | Phenomenex Strata X SPE | ACE Excel Super C18 | Gradient elution:1.0% of formic acid in water/ acetonitrile |
| 6 | Carglumic acid | 25 to 10000 ng/mL | PPT with Methanol | Agilent Zorbax SB-CN | 0.1% formic acid water/Methanol/Acetonitrile |
|
7
|
Levodopa Carbidopa |
10.0 to 5000.ng/mL 1.0 to 500 ng/mL |
PPT with Acetonitrile 1% TFA | Waters Xbridge Shield RP18 | Gradient elution:2% Acetic Acid in water/Methanol |
| 8 | 6-Mercaptopurine | 0.3 to 300 ng/mL | PPT with Methanol | Waters Xbridge Amide | Gradient elution: 2 mM ammonium formate and 0.2% acetic acid water/ Acetonitrile |
| 9 | Phenylpropanolamine | 2.0 to 2000 ng/mL | PPT with Acetonitrile | Phenomenex Luna CN | 5 mM ammonium trifluoroacetate water/Acetonitrile |
Chromatographic Techniques for Polar Compounds
Reversed-phase Chromatography:
Gradient elution is a robust separation tool to screen the retention characteristics of polar compounds using reversed-phase liquid chromatography (RP-LC).
Example: For quantitation of Morphine and its polar metabolite, Morphine-6-β-D-glucuronide, a 5.5 min gradient of 3-80% acetonitrile-water gradient was used and achieve a significant retention and resolution for both analytes with a 50 mm x 2.1 mm, 5 µm C8 column.
Adjustment of pH is another way to optimize the retention characteristics for a polar compound by RP-LC. Acidic or basic compounds usually require a pH-controlled mobile phase to maximize significant signal-to-noise, retention and resolution.
Example: During method development on the assay for vancomycin in human plasma, use of 1% of formic acid was added into mobile phase to maximize sensitivity and used an ACE Excel Super C18 analytical column.
Currently, there are a variety of polar-endcapped C18-based HPLC columns on the market capable for use under highly aqueous conditions. The bonding phases on these columns are end-capped with a proprietary polar group to provide superior retention for polar compounds via polar interactions, hydrogen bonding or electrostatic interactions.
Example: Phenomenex Luna Omega Polar C18 column was used for simultaneously determination of Amlodipine and Lisinopril in plasma. Where a Waters XBridge Shield RP18 column was used for retention and separation for Levodopa and Carbidopa.
Normal-Phase Chromatography:
Normal phase chromatography is the most frequently used separation technique for extremely polar compounds. Normal-phase separations depends upon polar adsorptive interactions. In general, for normal-phase liquid chromatography, the stationary phase is polar functional groups and the mobile phase is non-polar organic solvent. Polar molecules will have higher retention than non-polar compounds under normal-phase chromatography. In normal-phase chromatography, stationary phases are typically silica-based or organic moieties with cyano or amino functional groups.
Example An Agilent Zorbax SB-CN and a Phenomenex Luna CN analytical column were chosen for separation of Carglumic Acid and Phenylpropanolamine respectively.
Hydrophilic interaction liquid chromatography (HILIC):
The concept of hydrophilic interaction liquid chromatography (HILIC) was first introduced by Alpert in 1990. HILIC involves use of a hydrophilic stationary phase and a relatively hydrophobic mobile phase constituents. HILIC has been successfully applied to a wide variety of analytes, including small polar pharmaceutical compounds, antibiotics, biomarkers, amino acids and proteins.
Examples: N-Acetyl-D-Alanine as n-acyl-alpha amino acid is extremely polar with a LogP of -0.8, where 6-Mercaptopurine is also a polar compound having a LogP of -0.1. A Waters Atlantis Hilic Silica Column was used for determination of N-Acetyl-D-Alanine in human plasma, whereas Waters Xbridge Amide column was applied to method development of 6-Mercaptopurine in plasma sample.
Ion-pairing chromatography:
Ion-pairing chromatography has been used as an alternative for obtaining satisfactory retention of polar analytes. The ion-pairing reagent is an amphiphilic molecule with a hydrophilic head and a hydrophobic tail. An ion-pairing reagent is added to the mobile phase to improve chromatographic retention of analytes on the lipophilic stationary phase through the formation of neutral ion pairs.
Example: Heptafluorobutyric Acid is an example of ion-pairing reagent, it was added into the mobile phase to achieve a successful retention and separation of Trientine and N1-Acetyltriethylenetetramine on a Phenomenex Kinetex C18 column.
Sample preparation:
There are three sample preparation techniques are commonly employed to extract analytes out of biological matrices: (1) protein precipitation (PPT), (2) supported liquid extraction (SLE) or liquid-liquid extraction (LLE), and (3) solid phase extraction (SPE). For polar active pharmaceutical ingredient, PPT and SPE are more versatile techniques compared to SLE or LLE especially for analytes where they are difference in the relative extraction recovery (e.g a drug with a very polar metabolite). As shown in the table above, various bioanalytical methods for polar drugs were successfully developed, validated and used in sample analysis in BioPharma Services Inc (BioPharma Services) which utilize PPT and SPE as the preferred sample preparation technique.
Why Choose BioPharma Services?
Liquid chromatography-MS/MS methods have been applied for the quantification of various types of polar compounds in human plasma at BioPharma Services. The chemical properties of polar compounds present a great challenge in bioanalysis. Despite this, BioPharma Services’s bioanalytical team are skilled at performing bioanalysis to support HAP studies, phase I trials on new chemical entities and bioavailability/bioequivalence studies (BA/BE) for a variety of small molecules and peptides. Our scientists are subject-matter experts providing exemplary service in troubleshooting, solving complex problems, and take pride in delivering high quality and meaningful data to our customers.
Written by: Authors: Wu Pak (Anson) Kwan and Hongzhi Liu
Find out why BioPharma might be the right partner for you! Learn more about BioPharma Services and the wide array of bioanalytical services we provide.
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials 1/2a and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.



