Bioanalytical Method Development: Therapeutic Peptides
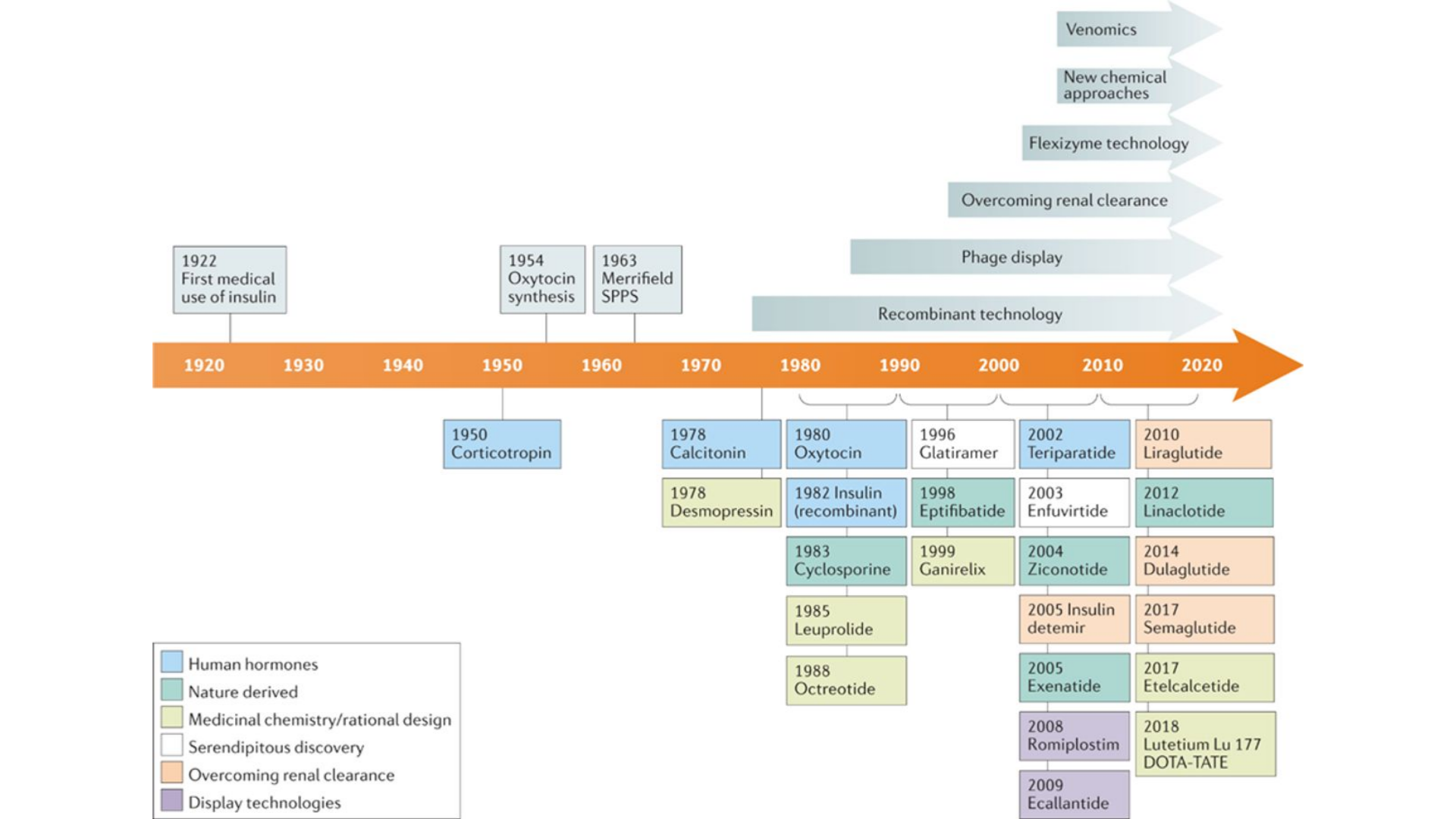
Therapeutic Peptides are short strings of amino acids, typically comprising 2–50 amino acids. The use of peptides as therapeutic (therapeutic peptides) agents started in 1922 with the first approval for the medical use of insulin. Currently, there are around 80 therapeutic peptide drugs on the market around the globe, and research into new therapeutic peptides continues at a steady pace.
Over 150 peptides are currently in clinical development and another 400–600 peptides undergoing pre-clinical testing. The global therapeutics peptides market was valued at approximately $39 billion (USD) in 2021 and, while reports vary, it is predicted to grow at a compound annual growth rate (CAGR) of approximately 5-10% from 2022 to 2030.
Use of LC-MS/MS in the analysis of therapeutic peptides
The development of bioanalytical methods for the quantitative analysis of peptides in biological matrices are crucial to the growing interest in novel therapeutic peptides and peptide biomarkers in biopharmaceutical research. Liquid chromatography–mass spectrometry detection (LC-MS, LC-MS/MS) has gradually become the method of choice for peptide bioanalysis over the past decade.
One of the significant advantages of an LC-MS/MS approach, over more traditional ligand binding assays, is that it does not require specific antibody reagents to achieve adequate selectivity to distinguish between other structurally similar peptides. The other benefits of LC-MS/MS for peptides analysis include broad linear dynamic analytical range; better accuracy and precision; efficient and lower costs for method development.
Biopharma Services Inc., has successfully developed and validated more than 250 LC-MS/MS-based assays for small molecules, that have been applied in the analysis of both clinical and non-clinical (GLP) clinical studies. Consistent with its planned expansion into larger/large molecule bioanalysis, a number of LC-MS/MS assays for therapeutic peptides have been developed in recent years that are the focus of this discussion piece:
Bioanalytical Method Development: Semaglutide
Semaglutide is a glucagon-like peptide-1 (GLP-1) analog that increases insulin secretion, thereby increasing sugar metabolism, and is indicated in the treatment of type-2 diabetes and long-term weight management.
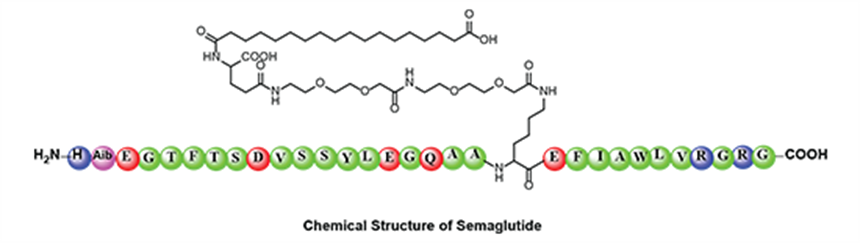
BioPharma Services bioanalytical assay utilizes a solid phase extraction procedure (Phenomenex SPE 96 well plate) requiring 200 μL of human plasma spiked with stable labeled internal standard (semaglutide-d8). Samples are chromatographically separated on a Waters C18 column using a gradient with Mobile Phase A (Acetic acid in water) and Mobile Phase B (100% acetonitrile with Acetic acid).
The mass spectrometer (AB Sciex QTRAP 5500) is operated in positive ion mode using a turbo Ion spray source and MRM detection. The total run time for a 25 μL injection is 7.0 minutes per sample. The assay was fully validated and has been successfully applied in clinical studies to assess the pharmacokinetics of Semaglutide.
Development of a Bioanalytical Method Development: Liraglutide
Liraglutide is another acylated glucagon-like peptide-1 (GLP-1) analog, similar to semaglutide and also used to treat type-2 diabetes, obesity, and chronic weight management.

Since Liraglutide and Semaglutide belong to the drug class, they have similar chemical and physical properties. The liraglutide assay utilizes the same SPE plate and LC column as the semaglutide method. The SPE wash and elute steps were modified to improve the recovery, LC gradient modified to avoid matrix effect. For improving the sensitivity, two MRM transitions were monitored and used for quantitation. The assay was fully validated and available for application in a biostudy.
Bioanalytical Method Development: Teduglutide
Teduglutide is a 33-amino acid polypeptide and classified as glucagon-like peptide-2 (GLP-2) analog used for the treatment of short bowel syndrome. It works by promoting mucosal growth and by restoring gastric emptying and secretion.
Glucagon-like peptide 2 (GLP-2) peptide molecule teduglutide
The bioanalytical assay developed by BioPharma Services utilizes a combination of protein precipitation and solid phase extraction procedure using a Phenomenex SPE 96 well plate. It requires 200 μL of human plasma spiked with stable labeled internal standard (teduglutide-d8). Samples are chromatographically separated on a Phenomenex C18 analytical column using a gradient with Mobile Phase A (water with acetic acid and tetrafluoroethanol) and Mobile Phase B (Acetonitrile with acetic acid and tetrafluoroethanol). The mass spectrometer (AB Sciex QTRAP 5500) is operated in positive ion mode using a turbo Ion spray source and MRM detection. The total run time per sample with a 30.0 μL injection is 7.0 minutes.
Key steps required for the successful development of an LC-MS/MS method for therapeutic peptides.
During the development of the bioanalytical assays for therapeutic peptides, a number of key considerations to ensure success were highlighted:
1. Understanding your peptide:
Knowledge of your peptide can help to predict potential problems and guide you towards a solution when challenges occur, such as (i) obtain the peptide sequence, (ii) calculate isoelectric point (pI), hydrophobicity index and retention characteristics on a reverse-phase HPLC column.
Keeping your peptide dissolved at each stage of sample handling (i.e., in solution, matrix and in extracted samples) is also critical. Inadequate or incomplete solvation of the peptide can cause poor linearity, poor and irreproducible chromatography, low sensitivity, and LC carryover etc.
2. Optimize liquid chromatography gradient profile:
Try to use slower flow rates, shallower gradients, and higher column temperature.
3. Choose an appropriate sample preparation procedure:
Solid phase extraction is a very effective sample clean up procedure for peptides compared to protein precipitation and liquid/liquid extraction. To minimize analyte loss, use of evaporation steps should be avoided in extraction procedures.
4. Select sensitive and specific MRM transition
The ultimate sensitivity and specificity of the method depends on the MRM transition selected. Generally, and within the instrument mass calibration range, the higher m/z precursor fragments will yield the best sensitivity. Choice of a fragment with m/z >precursor also provides better selectivity and specificity.
5. Minimize non-specific binding
Due to their amphiphilic properties, peptides, especially large peptides, often tend to be adsorbed to solid surfaces including storage tubes, pipette tips, injection syringes, etc. This can lead to poor recovery and loss of sensitivity, irreproducible chromatography, and non-linearity of standard curve. Non-specific binding should therefore be carefully evaluated and optimized at the beginning of the method development process.
6. Peptide stability in biological matrices
Lyophilized peptides are generally very stable, however, when they are placed in solutions or in biological matrices, peptides may be subject to chemical and enzymatic degradation [9]. Stabilizer may be need for reliable quantitative analysis.
Why Choose BioPharma Services for your next Clinical Trial Protocol Development?
Scientists and pharmaceutical companies believe therapeutic peptides represent a rapidly growing class of compounds indicated in a wide variety of therapeutic indications including chronic diseases such as cancer and metabolic disorders such as osteoporosis, obesity, and diabetes.
BioPharma Services Inc., has built on its years of research experience in the development of high-quality bioanalytical assays for small molecule drugs and successfully developed bioanalytical assays for a series of therapeutic peptides using LC-MS/MS detection. While therapeutic peptides possess their own unique bioanalytical considerations and challenges, when applied in the pharmacokinetic studies the developed methods still meet the exacting standards set at Biopharma Services, with low batch failure rate and high incurred sample reproducibility.
Find out why BioPharma might be the right partner for you! Learn more about BioPharma Services and the wide array of bioanalytical services we provide.
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials 1/2a and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.