Risk Management in Clinical Research: Minimizing risk and ensuring data integrity
Risk management in clinical trials is the forecasting and evaluation of risk together with the identification of procedures to avoid or minimize impact.
Translating that to the clinical research world of BioPharma Services, where studies are conducted with human subjects, means the “risk” is the potential compromise on the health and safety of the study participants, as well as the reliability of the study results. The process of risk management in clinical trials has immense impact. It is vital to identify, understand and manage the risk of clinical conduct and uncertainties that may occur from the emerging new drug discoveries.
To successfully carry out risk management in clinical trials, three key factors are discussed here.
Personnel and Qualification
-
- Clinical Staff: A Principal Investigator (PI) is the physician who leads the conduct of a clinical trial at a study site. The leadership role of the PI helps create the foundation of a successful clinical trial and is responsible for all clinical research activities, ensuring the study is conducted in accordance with national regulatory agency requirements and “Good Clinical Practice” (GCP). Compliance with GCP provides assurance that the rights, safety, and well-being of trial participants are protected, and that the results of the human clinical trials are factual and accurate.
- Principal Investigator: Supporting the PI, there is a team of study leads performing various aspects of a clinical conduct recruitment, screening, medical safety, study coordinators, sample managers, analytical leads, data managers, statisticians, and medical report writers. All ensuring the study conduct is performed as per GCP and study protocol design.
To ensure a smooth risk management in clinical trials, everyone is committed, trained, and qualified prior to being delegated to perform the respective task in the study by the PI. Qualification via academia, on the job training and/or relevant experiences are ensured.
Training is conducted in various formats; from independent information reading sessions to hands-on mock exercises are conducted. Training is managed in a 21 CFR part 11 electronic Quality Management System (eQMS). Training is delivered in a timely manner, monitored, and recorded in each employee’s training profile.
With our highly trained and experience team of scientific and medical expert is the true reflection of our mission, where “people matter” and together achieve success defined in the processes, controls, and study execution with minimal risk to the health & safety as well as the clinical study data integrity.
Study Participants
In the process of risk management in clinical trials, extending the ideology of “people matter” includes the study participants and the relationship we develop with them. Commitment or retention of the study participants is more effective with professionalism, and open information communication, sets a tone of friendliness and trust.
Understanding and trust here does affect the participant’s study compliance and subsequently on the overall health and safety aspects of the clinical study. Study participants need to understand the schedule commitment and objectives of the clinical study. To accomplish that, the Information Consent procedure is pivotal and should be conducted in compliance to E6(R2) Good Clinical Practice.
Study information and procedure is explained with opportunities for the study participants to request for clarity. The study participants should not be coerced into participation. Study participation should be a voluntary commitment.
Study participants are informed of:
- the known risk, discomfort, and benefits of the trial
- procedures and treatments involved
- Privacy and confidentiality
- Compensation and medical treatment in an event of an injury
- their rights to not participate or withdraw from a study, rights to be informed of any new emerging information when realized during study conduct.
Further assurances that the study participant’s health and safety is a priority in the process of risk management of clinical trials, the study information is reviewed and approved by an Independent Ethics Board (IRB). IRB has the authority to approve, modify or disapprove research in the interest of protection of human subjects in clinical trials. Contact information is provided in the event of research related query or injury to the study participants.
Study design and procedures
Clinical trials are classified into phases based on the objectives of the trial.
|
Phase I |
A phase 1 clinical trial is the first study conducted in humans using an experimental intervention. These trials often have small sample sizes (e.g., <20), may enroll healthy human participants, and are used to investigate pharmacokinetics, pharmacodynamics, and toxicity. |
|
Phase II |
Trials typically conducted to investigate a dose response relationship, identify an optimal dose, and to investigate safety issues. |
|
Phase III |
Generally large trials (i.e., many study participants) designed to “confirm” efficacy of an intervention. Sometimes called “confirmatory trials” or “registration trials” in the context of pharmaceutical development. |
|
Phase IV |
Trials carried out after registration of an intervention. They are generally very large and are typically conducted by pharmaceutical companies for marketing purposes and to gain broader experience with the intervention. |
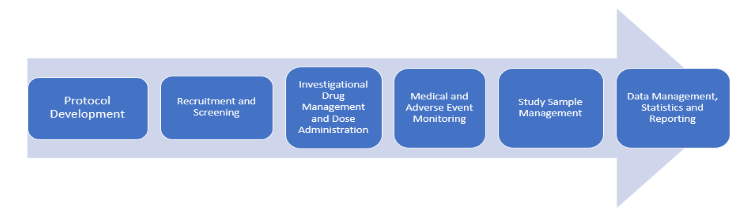
Clinical Trial Processes and Risk Mitigation Control
-
- Protocol Development
Once the study objective is determined and understood, the highly trained BioPharma Services project team of scientific and medical experts construct the study design, defining the following in a study protocol.
- study population,
- treatment course and duration,
- procedural conduct
- cost and logistics
- ethical considerations,
- statistical considerations
Risk considerations in the study design are evaluated and minimized via multiple levels of review from each subject-matter experts (SME) within the Clinical Research Organization (CRO), Sponsor and the Ethics Board. The study can only be conducted when all approvals are obtained, and No Objection Letter (NOL) is received from Health Canada.
2. Recruitment and Screening
Based on the approved study protocol, a recruitment plan defining the study population, recruitment methods and management is executed. Potential subjects are screened for their qualification requirements as per study protocol criteria, followed by the understanding of the study and confirmation to voluntarily participate via the Informed Consent process prior to enrolment to a study.
3. Investigational Drug Management and Dose Administration
Licensed pharmacists manage and prepare the investigation drug for dosing. They are labelled as per GMP requirements, dispensed as per study design’s approved randomization, blinding plan. Dosing is performed by a PI delegated staff and verified by another qualified personnel. The entire process is monitored under the supervision of the PI.
4. Medical and Adverse Events Monitoring
All study participants are continuously monitored, and treatment administered as needed by the study medical personnel. All events are followed-up to a resolution. Vitals (heart rate, respiratory rate, blood pressure), ECG, clinical safety samples are collected, and health of the study participants is monitored at a defined interval. Medical physical examinations are also performed prior to discharge from the study participation.
5. Study Sample Management
Based on predefined handling, processing, and storage study requirements, the sample integrity is managed by a qualified staff. Temperature, humidity and environment conditions are controlled during processing as well as shipping (if applicable), storage and analysis.
6. Data Management, Statistics and Reporting
All data (participant’s personal information and study data) are handled in compliance to
- the HIPAA (USA), PIPEDA (Canada) and Ontario provincial PHIPA privacy legislation
- Data Integrity FDA 21 CFR Part 11 requirements
- International Council for Harmonization (ICH) E6(R2) Good Clinical Practice
Qualified Pharmacokinetic scientists, data statisticians and medical writers, work on the collected information and present them in a form that has reasonable and accurate inferences and allow clinical researchers to make sound decisions with minimizing/preventing uncertainty errors and bias in medical research.
Why Choose BioPharma Services?
Overall, Biopharma Services Inc. takes all aspects, from people to its processes, into consideration in minimizing health and data integrity risk. The people conducting the clinical studies are trained and qualified, the study participants are treated ethically with safety in perspective, in every stage of clinical conduct the procedures are carried out keeping in mind all necessary aspects of risk management in clinical trials, but still maintaining regulatory compliance and due diligence to the ethics of medical research.
Find out why BioPharma might be the right partner for you! Learn more about BioPharma Services and the wide array of bioanalytical services we provide.
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials 1/2a and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.



