Clinical Trial Recruitment
Clinical Trial Recruitment is the process of identifying and enrolling suitable volunteers into a medical study. It’s a crucial step in testing new drugs or treatments, ensuring they are safe and effective before they’re available to the public. Successful recruitment can contribute to advancing medical knowledge and patient care.
The complexity of clinical trials requires efficient coordination of various activities related to their design, conduct, and completion. Clinical trial recruitment has been identified as one of the bottlenecks in clinical trial development. One study estimated that 11% of clinical research sites do not manage to enroll any participants, whereas 37% of clinical research sites under-enroll study volunteers.
Challenges in Clinical Trial Recruitment and Retainment
There are many challenges to the timely recruitment of a sufficient number of normal healthy volunteers (NHVs) or subjects for clinical trials. Even when clinical trial recruitment is successful, retaining study participants until the successful completion of a clinical trial may prove difficult. The underlying reasons are diverse and include:
-
- Information on upcoming clinical trials may not reach a sufficient number of eligible individuals.
- Potential study volunteers may have concerns regarding the risk of adverse effects of New Chemical Entities (NCEs) and Investigational Medicinal Products (IMPs) administered during the course of clinical trials.
- The accessibility of a clinical research site may be a concern for some potential study participants if they live far from the site or experience logistic challenges traveling to it.
- Prolonged study duration may place high demands on participants’ time, which may be unacceptable or unsustainable for a subset of them.
- Stringent eligibility criteria may limit the number of potential participants.
- Changes in participants’ circumstances may limit the time they have available for the study or create difficulties in commuting to the clinical research site.
- A subset of study participants with a medical condition may be concerned that they will receive a placebo or an active treatment that is less effective than standard therapies during the course of a clinical trial. In addition, some study volunteers may not perceive any benefits from the NCE or IMP they receive as a part of the clinical trial, which may weaken their motivation for participation.
Negative Effects of Inefficient Recruitment on the Outcomes of Clinical Trials
There are many negative consequences of inefficient subjects recruitment in clinical trials. Thus, inadequate recruitment may prolong the duration of clinical trials, which, in the long run, may delay the public’s access to novel therapeutics. Not being able to recruit a sufficient number of study participants may also negatively affect the statistical power of clinical trials, increasing the risk of a type II statistical error. Delays in volunteer recruitment may increase the cost associated with clinical trials.
Improving NHV & Subjects Recruitment in Clinical Trials
Different strategies can be used to increase the efficacy of subjects recruitment in clinical trials. They include:
Expanding the Outreach to Study Volunteers
Extending the outreach to more potential study volunteers via the press, media, and online resources may help recruit more study participants. Creating positive awareness of the clinical trial may also increase the recruitment rate. In addition, networking with other medical professionals may help reach even more potential study participants. Finally, a database of potential study participants may serve as an important additional source for clinical trial recruitment.
Providing Detailed Information to Study Volunteers
Informing potential participants about the clinical trial procedures in sufficient detail may help relieve their anxiety and concerns and enhance the rates of their participation and cooperation.
Design of an Efficient Recruitment & Retention Plan Tailored to Study Needs
Planning how to recruit and retain study participants while considering the study’s specific characteristics may help enhance the efficiency of clinical trial recruitment. A comprehensive recruitment strategy should be developed before the start of the recruitment process, considering the characteristics of the participants, factors motivating their participation, and barriers to their involvement in the study. However, the recruitment strategy should also be adaptive to enable responsiveness to incoming data or changes in participants’ circumstances.
Facilitating Participants’ Access to the Study Site
Helping study volunteers overcome logistic challenges regarding their access to the study site, such as providing travel assistance, may elevate the rate of participants’ compliance with the study protocol.
Building Strong Rapport with Study Volunteers
Establishing a solid relationship with study volunteers built on mutual respect and trust is a prerequisite for efficient communication throughout the trial and may enhance their adherence to the study procedures. Clinical trial research coordinators play an important role in strengthening the rapport with study volunteers.
Identifying Barriers to Study Participation
The timely identification of barriers to study recruitment and retention and the design of strategies to overcome them are also valuable strategies for enhancing compliance with the trial procedures. Open communication with study participants is a prerequisite to achieving this goal.
Establishing Participant Databases as a Strategy to Optimize Clinical Trial Recruitment
One of the essential strategies to streamline NHV and subjects recruitment for clinical trials is the establishment of participant databases. There are many advantages of this approach. First, volunteer databases include individuals interested in participating in a clinical study. Moreover, preregistered volunteers may be recruited not only once but also for multiple clinical trials. Finally, individuals included in the volunteer databases have been pre-screened, which means that some of their data are already available, and the subsequent clincial trial recruitment process will be more straightforward.
BioPharma Services’ Expertise in Clinical Trial Recruitment
An Extensive Database of Potential Study Volunteers
We have established a comprehensive database encompassing over 18,000 individuals. Our database includes the following:
-
- NHVs
- Recreational drug users, including individuals consuming opioids, cannabinoids, and stimulants
- Post-menopausal and surgically sterile females
- Specialty ethnic groups, such as Caucasians and Asians
Focusing on Volunteer Safety as our Highest Priority
We strictly adhere to the United States Food and Drug Administration (FDA) and Health Canada’s recommendations regarding clinical trial recruitment and during all stages of clinical trial design and conduct. In addition, our organization developed an efficient response strategy to coronavirus disease 2019 (COVID-19), which enabled us to continue operations safely and efficiently during the COVID-19 pandemic.
State-of-the-art Phase 1 Clinical Trial Center
Our clinical research facility in Toronto, Canada, has been designed to ensure the safety of study participants throughout clinical trials. It contains dedicated Phase 1 intensive monitoring units, 24-h monitoring, nursing stations in the rooms, a telemetric option, and a crash cart. The team operating our Phase 1 clinical trial center is ALCS-trained and includes highly qualified and experienced principal investigators (PIs), anesthesiologists, and emergency room (ER) physicians.
An Experienced Team of Recruitment Professionals
BioPharma Services’ team has extensive expertise in recruiting study participants for early-phase clinical trials and establishing a strong rapport with them. In addition, our clinical trial recruitment specialists collaborate closely with other experts from our team, including safety physicians, to ensure that eligible study participants are recruited for each study and that their safety is ensured throughout the clinical trials.
Our Recruitment Process
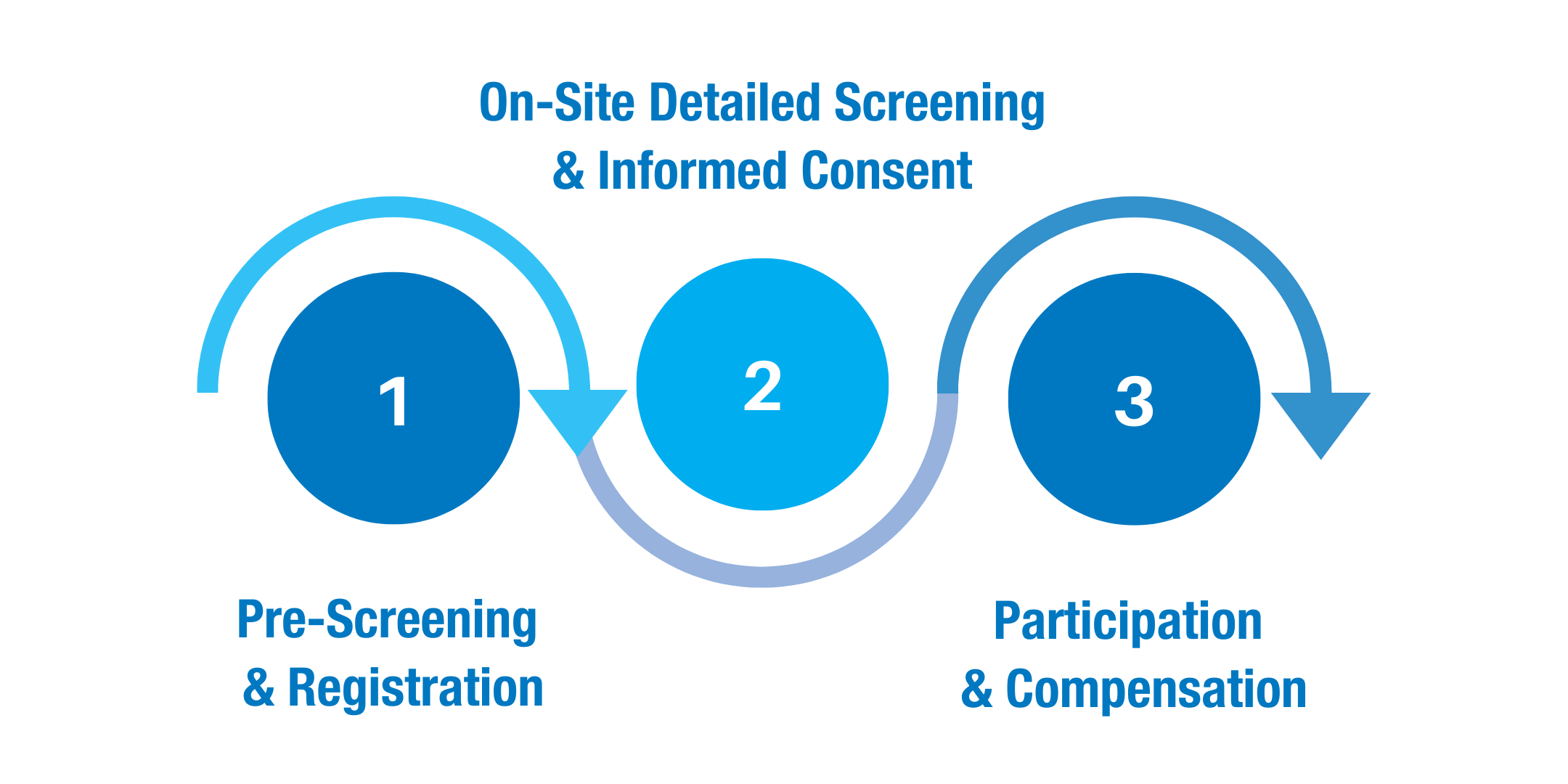
BioPharma Services has established a well-structured and efficient clinical trial recruitment process that enables the recruitment of eligible participants.
-
- A registration/pre-screening is conducted to inform potential study participants about upcoming clinical trials and to preliminarily screen them for eligibility.
- After the registration is completed, a visit to one of BioPharma’s study sites follows. There, participants are screened in detail for their eligibility for the study. Medical assessments may include determining the body mass index (BMI), blood pressure, respiratory and heart rate, and body temperature. Further, an electrocardiogram (ECG), a physical examination, a medical history review, an alcohol test, blood tests, and urine tests are performed. The potential study participants undergo an in-person consent session during which they are informed about all aspects of the planned clinical trial, including potential study risks.
- Subsequently, the study volunteers complete the study visits specified in the protocol and are compensated for their participation.
Ready To Get Started?
Complete the Form below to schedule a Discovery Call with a member of our medical team to learn how BioPharma Services can be your trusted Clinical Trial Recruitment partner!
Schedule a Discovery Call
You can unsubscribe at any time. For more details, please read our Privacy Policy.
Clinical Trial Recruitment FAQ
-
What Are Three Challenges to Subject Recruitment in Clinical Trials?
Three common challenges to patient recruitment in clinical trials are:
- Low awareness of the trial: Many potential subjects may not know about the trial, its potential benefits, or how to enroll.
- Ineligibility of potential subjects: Some potential subjects may not meet the eligibility criteria for the trial, which can limit the pool of eligible participants.
- Lack of interest or motivation: Even if a potential subject is eligible and aware of the trial, they may not be motivated or interested in participating.
-
Why is Clinical Subject Recruitment Important?
Subject recruitment is critical to the success of a clinical trial. Adequate subject recruitment ensures that the trial can be completed on time and within budget, which is important for meeting regulatory and business objectives. It also helps to ensure that the trial results are statistically valid and can be used to support regulatory submissions and product approvals. Additionally, subject recruitment is important to ensure that the trial represents a diverse population, which can improve the generalizability of the trial results.
-
What are Sponsor Responsibilities for Clinical Trial Subject Recruitment?
As per Good Clinical Practice guidelines (GCP), sponsors are responsible for the following aspects of subject recruitment in clinical trials:
- Developing the recruitment strategy: Sponsors should develop a recruitment plan that outlines how potential subjects will be identified, approached, and enrolled in the trial.
- Ensuring informed consent: Sponsors should ensure that potential subjects are provided with all the necessary information about the trial and that they provide informed consent before enrolling.
- Monitoring recruitment progress: Sponsors should regularly monitor the recruitment progress and make adjustments to the recruitment plan as needed.
- Ensuring subject safety and well-being: Sponsors should ensure that the rights, safety, and well-being of the subjects are protected throughout the trial.
-
What are Some Ethical Considerations with Subject Recruitment and How Does a BioPharma Services Ensure Ethical Subject Recruitment Practices?
Ethical considerations with subject recruitment in clinical trials include informed consent, protection of vulnerable populations, and ensuring that participants are not coerced into participating. BIoPharma Services ensures ethical subject recruitment practices through adherence to guidelines and regulations set forth by organizations such as the International Conference on Harmonization (ICH) and the World Medical Association. We work closely with our sponsors and clinical sites to ensure that informed consent is obtained appropriately, and that patients are fully aware of the potential risks and benefits of participation. We also prioritize the safety and well-being of all study participants and ensure that vulnerable populations, such as children and pregnant women, are not put at risk. Additionally, we work to avoid any undue influence or pressure on potential participants and ensure that their decision to participate is fully voluntary.
-
What Criteria Are Used for Subject Selection in Clinical Trials?
The criteria for subject selection in clinical trials are outlined in the study’s protocol and typically include factors such as age, gender, type and stage of a treatment, previous treatment history, and other medical conditions. These criteria are not used to reject people personally, but rather to identify appropriate participants who will most likely benefit from a clinical trial and those who could have negative outcomes due to the trial conditions. It is also used to ensure that the study results will be relevant and reliable.
