Sample Size Estimation and Passing Rate Analysis for Highly Variable Drugs Using FDA’s Scaled Average Bioequivalence Approach
PRESENTED TO: BioPharma Services Inc.
PRESENTED BY: N. RAYAD, N. GHARAVI, J. HE, F. TRABELSI
PURPOSE
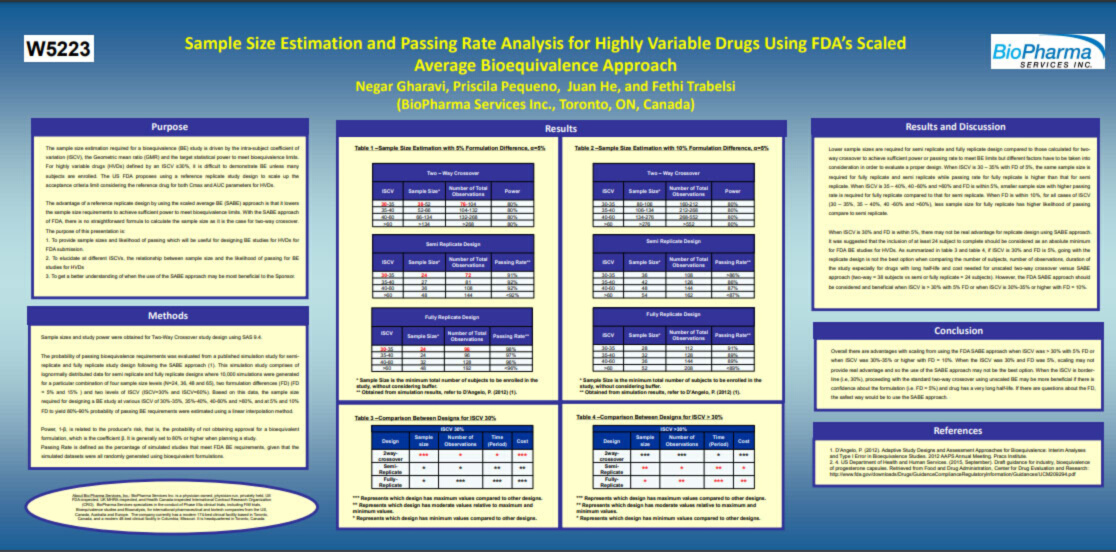
Sample Size Estimation and Passing Rate Analysis for Highly Variable Drugs Using FDA’s Scaled Average Bioequivalence Approach – The sample size estimation required for a bioequivalence (BE) study is driven by the intra-subject coefficient of variation (ISCV), the Geometric mean ratio (GMR) and the target statistical power to meet bioequivalence limits.
For highly variable drugs (HVDs) defined by an ISCV ≥30%, it is difficult to demonstrate BE unless many subjects are enrolled. The US FDA proposes using a reference replicate study design to scale up the acceptance criteria limit considering the reference drug for both Cmax and AUC parameters for HVDs.
To access the entire publication and view the full resolution PDF, please complete the form.