An Effective Approach to Assess Bioequivalence of Fingolimod in Normal Healthy Volunteers Using 0.5 mg Study Dose
PRESENTED TO: BioPharma Services Inc.
PRESENTED BY: R. Chandani, J. He, F. Trabelsi
PURPOSE
Fingolimod is indicated for the treatment of patients with relapsing forms of multiple sclerosis (MS) to reduce the incidence of clinical exacerbations, as well as to delay the progression of physical disability. The recommended dose of fingolimod is 0.5 mg orally once daily, although the FDA product-specific recommendation for generic drug development suggests a dosage strength of 0.5 mg x 3 capsules (1.5 mg dose).
Furthermore, the FDA label for fingolimod states that dosage strengths above 0.5 mg have been associated with greater occurrence of adverse reactions, without any significant additional therapeutic benefit. Although a 1.5 mg dose would certainly produce robust concentration values upon sample bioanalysis, an appropriately sensitive analytical method calibrated to a 0.5 mg dose would allow to administer a low dose to healthy volunteers, and would be closer to the therapeutic once daily dose of fingolimod.
Several studies have demonstrated that fingolimod is efficiently absorbed with oral bioavailability of >90%, and can be taken with or without meals. Fingolimod demonstrates a doseproportional exposure relationship between 0.125- 5 mg doses, and low intra-subject variability (<20%). Fingolimod demonstrates a terminal elimination half-life of 6-9 days, and as a result an adequate washout period is required to mitigate the risk of significant carry over effects, while maintaining practical study conditions. The FDA product specific guidance for drugs with long elimination half-fie (>24h) recommends a singledose crossover study is sufficient provided an adequate washout-period is implemented, and if not, a parallel design may be used. Based on a half-life of 6-9 days, a washout period of 6-10 weeks is suggested for cross-over design conditions to minimize the risk of pre-dose values in subsequent dosing periods, or carry-over effect.
METHOD(S)
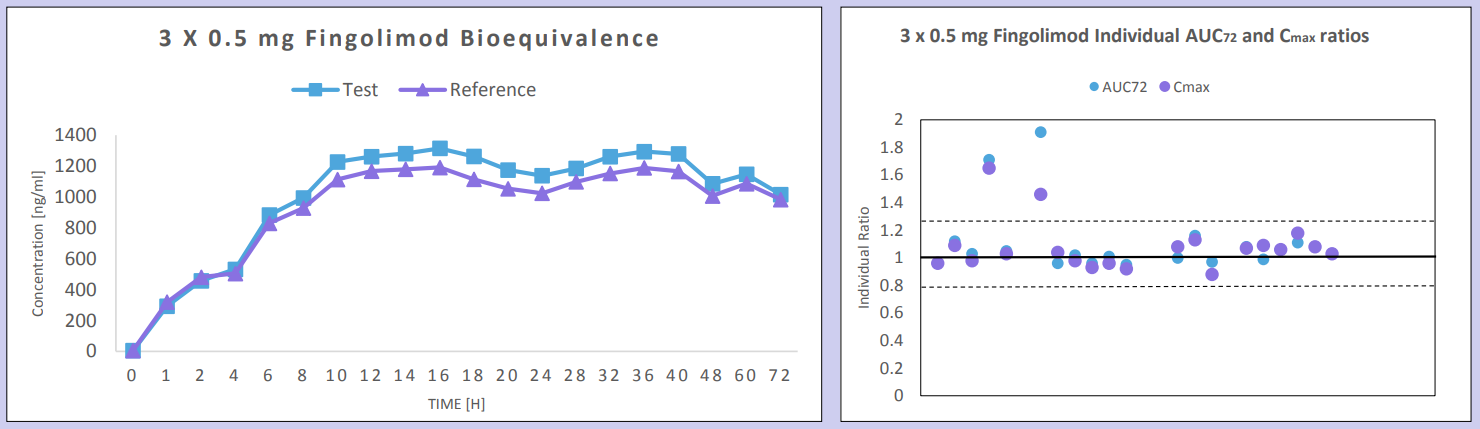
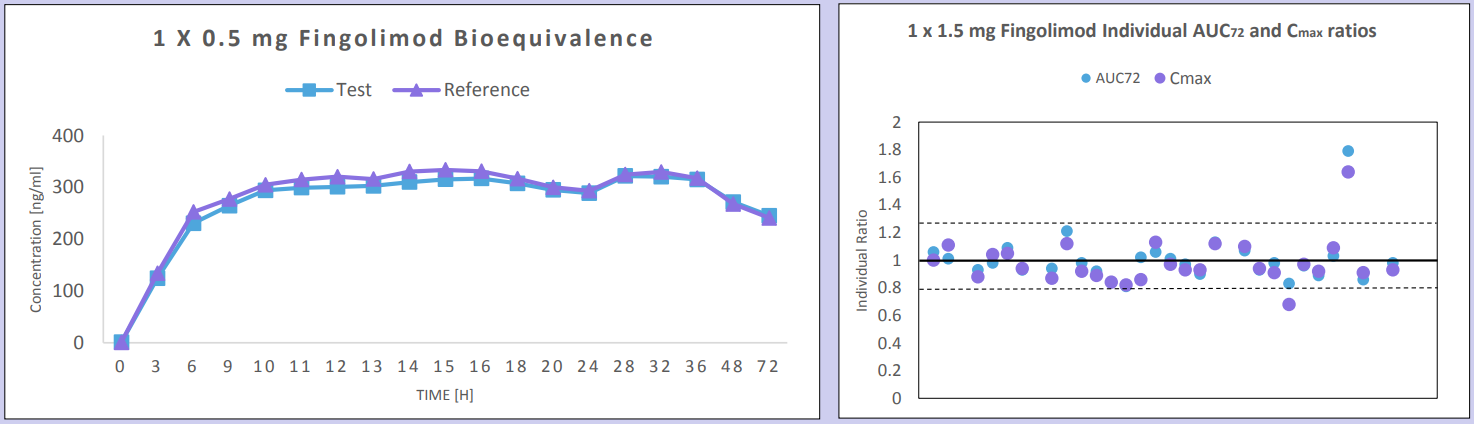
Seven BE studies were conducted using 1 x 0.5 mg capsule strength doses of test and reference treatment products of fingolimod in normal healthy volunteers; 4 under fasting conditions, and 3 under fed conditions. Four of these studies were conducted with a wash-out period of 6 weeks, one study with a wash-out period of 7 weeks, one with a wash-out period of 10 weeks, and one study was conducted parallel design and thus there was not any wash-out period required. In addition, two BE studies were conducted using 3 x 0.5 mg (1.5 mg) strength doses; 1 under fasting, and 1 under fed conditions.
RESULTS
Bioequivalence criteria was met in all 7 studies conducted using 0.5 mg x 1 capsules of test and reference treatment products of fingolimod in normal healthy volunteers with wash-out periods of 6-10 weeks or in parallel design. Pre-dose concentration values were observed in each of the six single-dose crossover studies which were >5% of Cmax; data from these subjects were excluded from the PK stats analysis as per FDA guidelines. Only one subject demonstrated a high pre-dose value >5% of Cmax in the study with a 10 week washout. Intra-subject variability was <20% in each study, and thus consistent with reported literature values for 1.5 mg dose.

CONCLUSION
With an appropriately sensitive analytical method, a dosage strength of 0.5 mg x 1 capsule was sufficient to assess bioequivalence of fingolimod in healthy volunteers. Ideally the washout period for fingolimod should fall within a 6-10 week range, however even with a 1/3rd reduction of dose strength from what is recommended in the FDA product specific draft guidance, several pre-dose values had been observed in subsequent dosing periods using cross-over design. Therefore, assessing bioequivalence using 0.5 mg dose is optimal in minimizing risk of carry-over effect under cross-over design, and is not a challenge if a sensitive analytical method is implemented.
The cross-over design allows for a reduced sample size, while bolstering statistical power, and increasing accuracy of ISCV estimation compared to parallel design
REFERENCES
1. Novartis pharma. “Highlights of prescribing information for Gilenya (fingolimod) capsules, for oral use.” Revised Feb 2016. Retrieved from http://www.accessdata.fda.gov/drugsatfda_docs/label/2016/022527s018lbl.pdf
2. FDA product specific draft guidance. “Fingolimod.” Recommended: Aug 2011. Retrieved from http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm270382.pdf
3. David, Olivier J., John M. Kovarik, and Robert L. Schmouder. “Clinical pharmacokinetics of fingolimod.” Clinical pharmacokinetics 51.1 (2012): 15-28



