Safe and Effective Design of Bioequivalence Study of 400 mg of Imatinib in Normal Healthy Volunteers under Both Fasting and Fed Conditions: A Biopharma Services Inc. Perspective
PRESENTED TO: BioPharma Services Inc.
PRESENTED BY: J. He, N. Gharavi, H. Somasundaram, U. Patel, R. Chandani, F. Trabelsi
PURPOSE
Imatinib is a small molecule protein-tyrosine kinase inhibitor which is used for the treatment of leukemia and stomach tumors. The Food and Drug Agency (FDA) product-specific guidance for bioequivalence (BE) assessment of imatinib mesylate recommends study strength/dose of 400 mg in patients who are already receiving a stable 400 mg dose, rather than normal healthy volunteers (NHV). In contrast to the FDA, the European Medicines Agency (EMA) has recently issued a product specific Bioequivalence guidance on imatinib recommending the use of NHV as the study population. EMA considers that a single dose of 400 mg of imatinib could be safely administrated to NHV.
Following extensive safety discussion and investigation, BPSI has conducted many studies with 400 mg imatinib in NHV. The objective of this poster is to share BPSI’s experience from a safety and pharmacokinetic (PK) perspective.
RESULTS
In total, approximately 270 NHV subjects were dosed with 400 mg imatinib, all AEs were either mild or moderate in nature. The assessment of likelihood that an AE was related to imatinib indicated that over 50% of the AEs were not caused by imatinib. The most commonly reported AE was sleepiness (<3%) in all studies. Other common AEs were headache, nausea and vomiting. Among all dosed subjects, there were 6 subjects (2.2%) who did not complete the study due to AEs. There were no clinically significant abnormalities in laboratory values, vital signs or ECG values. Overall imatinib was well tolerated by NHV.
| Total Subjects | Total AEs | Subjects with mild AE | Subjects with moderate AE |
| 270 | 201 | 187 | 14 |
| AUCt (ng.h/mL) | AUCinf (ng.h/mL) | Cmax (ng/mL) | Tmax (h) | T1/2 (h) | |
| Test (Fed) | 34434 | 35731 | 1900 | 4 | 15.56 |
| RLD (Fed) | 36230 | 37080 | 2031 | 4 | 15.48 |
| Imatinib | Cmax | AUC |
| Fasting | < 15% | < 15% |
| Fed | 15 to 20% | < 15% |

METHOD(S)
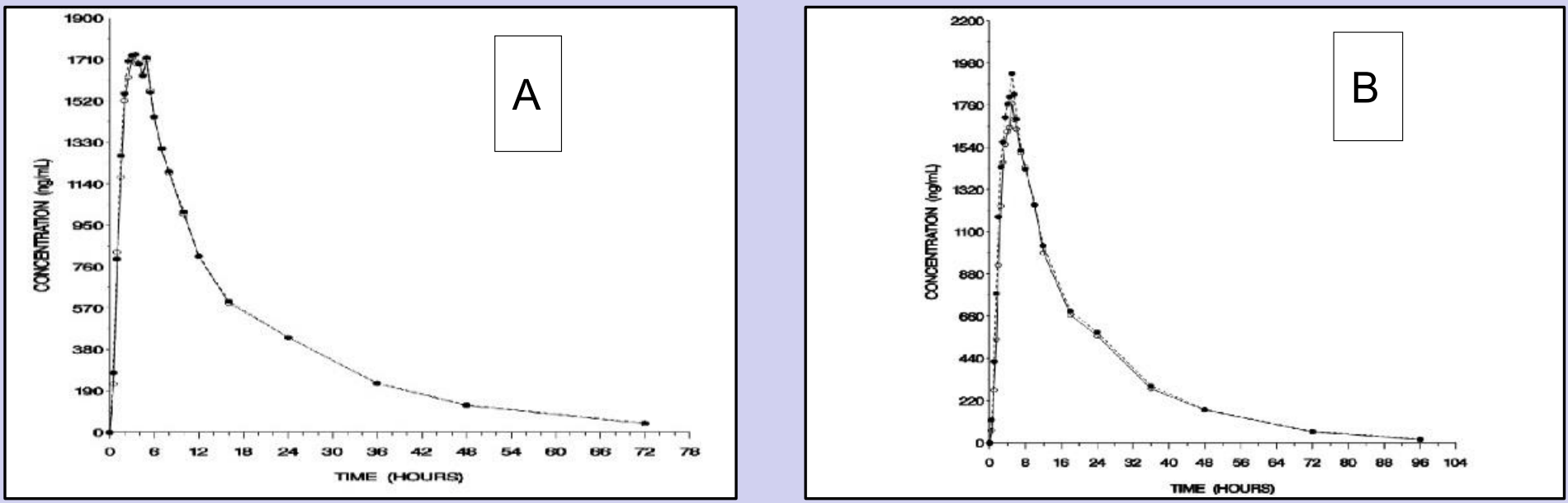
Ten (10) pivotal BE studies were conducted in NHV for potential EMA and FDA submissions. These trials were designed as open-label, randomized, two sequence, two-treatment, two-period, crossover studies conducted either under fasting or fed conditions.
There were 2 studies conducted under fasting conditions and 8 were conducted under fed. All subjects were normal healthy males and non-pregnant females aged 18 years and older, with a body mass index between 18.5-30.0 kg/m2. All eligible subjects received a single oral dose of 400 mg of both test and reference products on separate occasions with 2 weeks of washout. A series of blood draws after drug administration (over a period of 96 hours) were collected for imatinib to establish individual PK profiles and PK parameters (e.g. Cmax, AUCt and AUCinf).
Safety observations (e.g. vital signs) were assessed by the principal investigator and/or qualified staff following a pre-defined schedule such as predose, 2, 4, and 24 (clinic exit) hours; and adverse events (AE) were assessed throughout the course of the study.
The primary PK parameters, maximum plasma concentration (Cmax) and area under the plasma concentration-time curve (AUC), were estimated and BE assessment was carried out with 90% confidence intervals approach when applicable.
CONCLUSION
Overall, the 400 mg strength imatinib was proven to be well tolerated by NHV and no SAE was reported following the single dose design from all studies conducted at BPSI.
Our in house data showed that the PK of imatinib in NHV demonstrated Tmax and half-life consistent to values reported in the product monograph.
Our results also showed that imatinib exhibits low intra-subject variability which suggests a low sample size is needed. Assuming high confidence with the generic drug prototype, sponsors do not need to evaluate the Test performance in a pilot but rather go ahead with Pivotal studies.
BE study of imatinib to be done in patients already receiving the 400 mg imatinib recommended by FDA was mostly due to the safety consideration. Our large experience with imatinib demonstrated that a single oral dose of 400 mg of imatinib in a two-way crossover study with NHV is safe and appropriate bioequivalence study design for for submissions indented for all regulatory agencies including the US FDA.
REFERENCES
1. EMA product specific guidance. “Imatinib hard capsules 50 and 100 mg, film-coated tablets 100 and 400 mg product-specific bioequivalence guidance* .” Recommended: Oct. 2013. Retrieved from http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/05/WC500207097.pdf
2. FDA product specific draft guidance. “Imatinib Mesylate .” Recommended: July 2014. Retrieved from http://www.fda.gov/downloads/Drugs/GuidanceComplianceRegulatoryInformation/Guidances/ucm118261.pdf



