Clinical Trial Data Management Services
Leading Clinical Trial Data Management Services
A clinical trial does not end with successful execution of the study. Of equal importance is the clinical trial data management of the data produced by the clinical research study. The precise, reliable and accurate collection of data organized and packaged to meet current regulatory and industry standards is imperative for every study. BioPharma’s Clinical Trial Data Management team is involved right from the outset to ensure that data is correctly managed every step of the way.
BioPharma‘s onsite Phase 1 Clinical Trial Data Management team is constantly looking for innovative and creative solutions to expand and improve on our clinical trial services’ processes and procedures that will allow us to exceed client expectations. We understand that Clinical Trial Data Management is so much more than simply collecting data electronically. From data collection to data review, query management and mapping under Study Data Tabulation Model (SDTM) standards and CDISC conversion, our goal with our clinical trial services is to hand over high quality study data to our clients in the most expeditious manner. Learn more about our team.

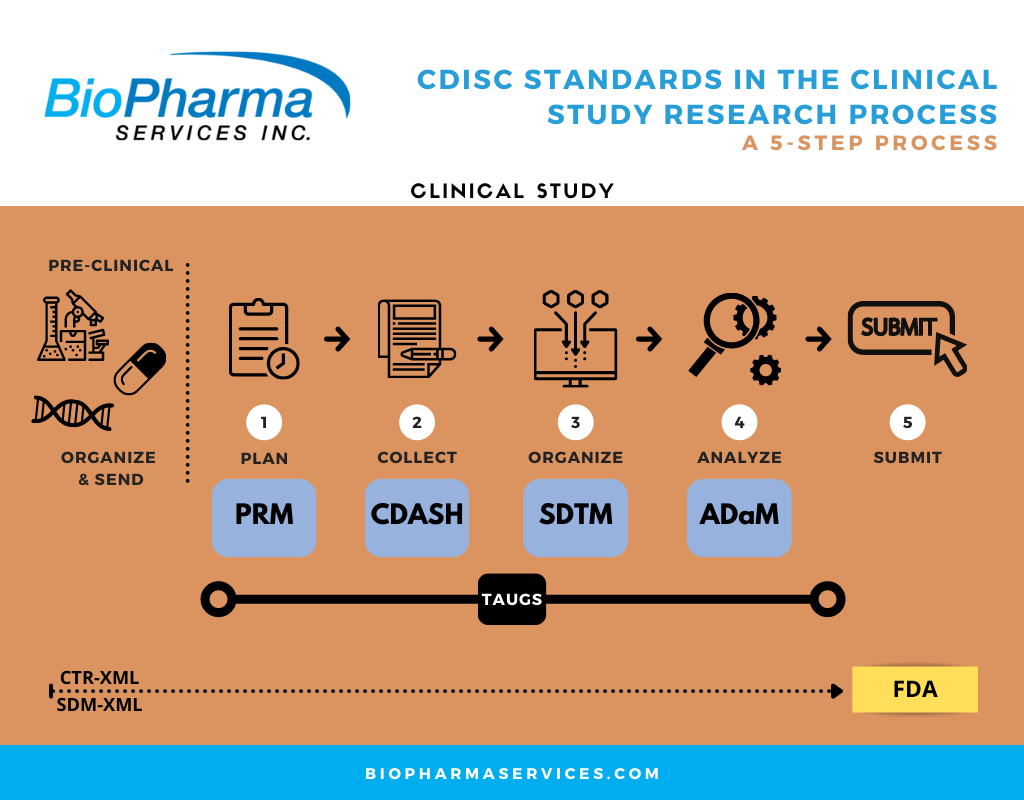
BioPharma’s CDISC Data Conversion Services
Conversion of clinical and metadata to comply with the Clinical Data Interchange Standards Consortium (CDISC) ensures compliance with regulatory requirements and streamlines the review process.
We support our clients with dataset mapping under Study Data Tabulation Model (SDTM) standards and CDISC conversion. Our team has expertise in creating SDTM annotated CRFs, SDTM datasets, ADaM analysis datasets, pooled analysis datasets (ISS/ISE), and data handling reports.
BioPharma Services specializes in Biostatistics and Data Management Services. Read more Here about BioPharma’s CDISC Data Conversion Services as part of your next Phase 1, Bioequivalence or Human Abuse Potential (HAP) study.
BioPharma’s full offering of Data Management for clinical trial services includes:
- Data Management Plan (DMP) development
- eCRF design and development
- Database Build & Design
- Data validation specifications
- Clinical Trial Data Management
- Edit checks Programming & Testing
- Collection of pharmacodynamics data and review
- Single or double data entry
- EDC Training
- eCRF Completion Guidelines
- Query Management
- Third-party data integration
- Customized clinical data management programming
- Safety Data Management & Reconciliation
- Data Export/Transfer
- Interim Analysis readiness
- Medical coding (CM, AE, MH)
- Serious Adverse Event (SAE) reconciliation
- Customized status reporting and data listings
- Database closeout and delivery
Our comprehensive CDISC data conversion services include:
- SDTM annotated CRFs
- SDTM datasets
- ADaM analysis datasets
- Pooled analysis datasets (ISS/ISE)
- Define.xml metadata and Define.pdf
- CDISC controlled terminology
- Data Handling report
- FDA reviewers’ Guide
- eCTD structure
Schedule a Discovery Call
Learn how BioPharma Services can be your trusted clinical trial partner.
