Bioequivalence and Bioavailability Studies
Industry Leading Bioequivalence (BE) Services
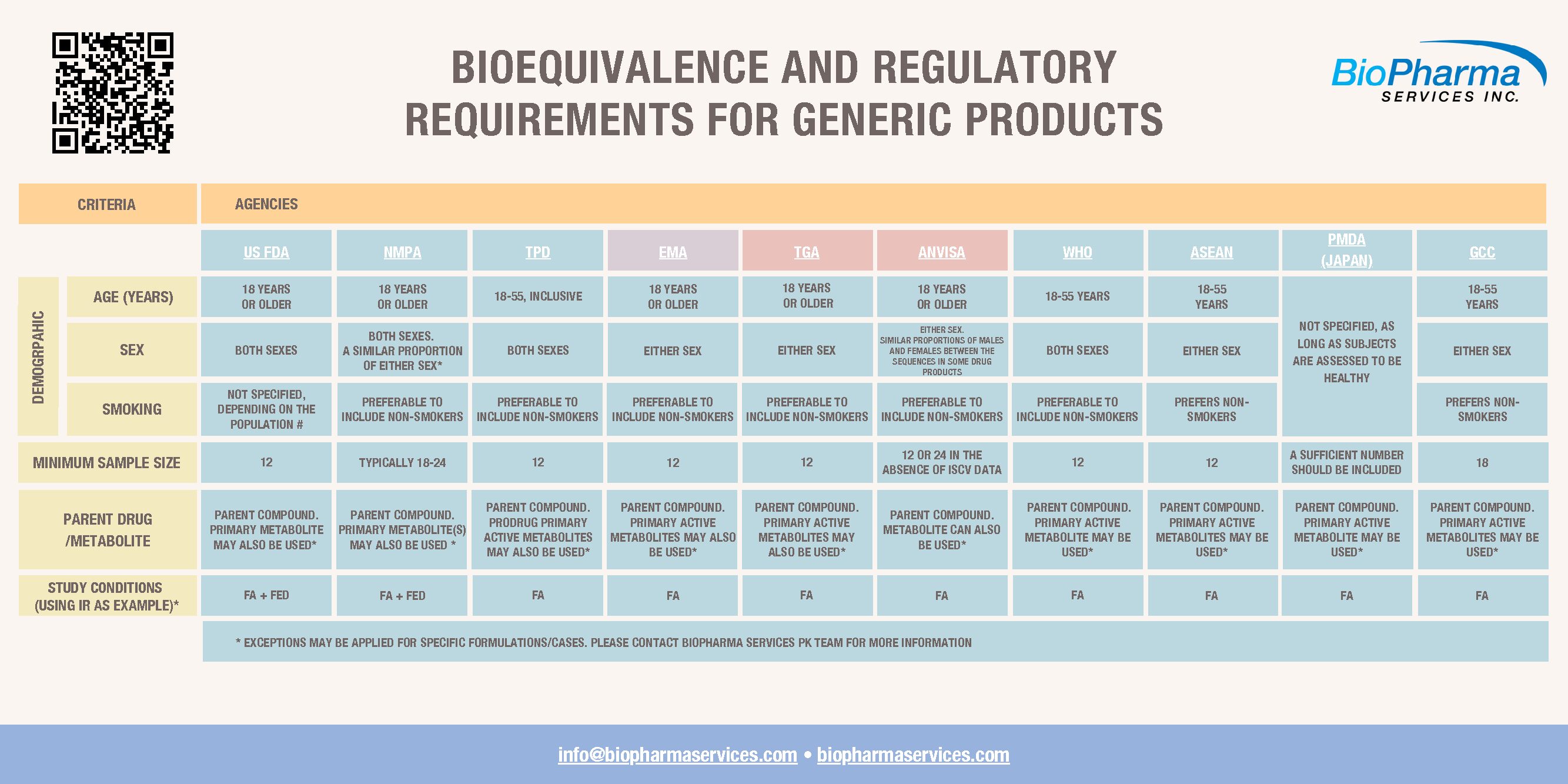
Generic drugs account for almost eight of every 10 prescriptions filled in the United States and cost approximately 80-85% less than their brand-name counterparts. Notably, generic drugs have to comply with strict regulatory Bioequivalence requirements for generic products regarding their bioequivalence to the corresponding brand-name drug, bioavailability, and overall quality.
The complexity of the regulatory process for generic drug approval is illustrated by the high number of Product-Specific Guidances for Bioequivalence Drug Development posted or revised by the U.S. Food and Drug Administration (FDA) both for drugs and complex products, such as metered dose inhalers during 2021.
BioPharma Services Inc., a full-service Clinical Research Organization (CRO) that has successfully completed over 2,000 clinical trials, offers first class bioequivalence services and comprehensively yet efficiently supports our clients with their bioequivalence and bioavailability studies and the development of our generic products.
Talented Multidisciplinary Team
Our accomplished team of clinical investigators, pharmacokinetic scientists, and clinical operation specialists has vast experience with all stages of bioequivalence and bioavailability studies. Moreover, our bioequivalence and bioavailability team works in close collaboration with our talented bioanalytical scientists, statisticians, and regulatory professionals to comprehensively support all aspects of our clients’ bioequivalence and bioanalysis programs.
State-of-the-Art Facility & Large Database of Study Volunteers
BioPharma Services has a state-of-the-art first in human clinical trial site located in Toronto, Canada, with all necessary infrastructure and expertise for phase 1 clinical trials, first in human studies and bioequivalence and bioavailability studies to support both generic and hybrid drug filings and 505(b)(2) New Drug Applications. In addition, BioPharma has established a volunteer database with over 18,000 potential study participants, including both male and female healthy volunteers and special populations, that enables fast and efficient recruitment.

Experts in Data Submission
Read and share this infographic (PDF, 137K)
To Learn More about the Bioequivalence and Regulatory requirements for generic products, speak with one of our industry leading experts.
We have extensive expertise in the development and evaluation of a wide range of drug formulations. We can competently and efficiently conduct studies on:
- Solid oral formulations
- Solid oral dosage forms for immediate release, extended release and modified release
- Orally disintegrating tablets
- Solid liquid formulations
- Inhalation products
- Transdermal patches
- Long-acting depot injections
- Creams, suppositories and ointments

Outstanding End-to-end Service for Bioequivalence and Bioavailability Studies
BioPharma can competently support the complete cycle of bioequivalence and bioavailability studies from bioequivalence study design and protocol inception to the issuance of the final clinical study report.
Competent and Efficient Study Execution
Our accomplished team of pharmacokinetic scientists, clinical operation specialists, and clinical investigators work together to ensure the competent and timely execution of bioequivalence and bioavailability studies that adhere to the principles of Good Clinical Practice (GCP).
Strict Quality Control
BioPharma’s quality assurance experts and quality control processes warrant the acquisition of high-quality scientific data and excellence of the conducted clinical research.
Competent In-House Bioanalysis of Samples Derived From Bioequivalence Studies
Our in-house bioanalytical laboratory enables the rapid and reliable analysis of samples derived from bioequivalence and bioavailability studies.
Statistical Support
Our team of accomplished biostatisticians provides excellent biostatistical services and programming support for our clients’ generic drug development programs.
Regulatory Guidance
BioPharma’s regulatory professionals ensure that our clients’ bioequivalence and bioavailability studies comply perfectly with regulatory bioequivalence requirements for generic products. Our in-house scientific and regulatory expertise is illustrated by BioPharma’s successful completion of numerous regulatory inspections from the FDA, Health Canada, European Medicines Agency (EMA), UK Medicines and Healthcare products Regulatory Agency (MHRA), Brazilian National Health Surveillance Agency (ANVISA), World Health Organization, French National Agency for the Safety of Medicines and Health Products (ANSM), Danish Medicines Agency (DKMA), and Standards Council of Canada.
Schedule a Discovery Call
You can unsubscribe at any time. For more details, please read our Privacy Policy.
Bioequivalence FAQ
-
What is a Bioequivalence study?
First, we would need to describe the definition of Bioavailability and Bioequivalence.
Bioavailability (BA): is a measurement of the rate and extent to which a therapeutically active chemical is absorbed from a drug product into the systemic circulation and becomes available at the site of action. For most drugs that are taken orally, the active ingredients are released in the gastrointestinal (GI) tract and arrive at their site of action via the systemic circulation. Bioavailability is assessed using two main pharmacokinetic variables: the area under the blood concentration versus time curve (AUC), and the maximum blood concentration (Cmax).
Bioequivalence (BE): If two drugs are bioequivalent, there is no clinically significant difference in their bioavailability. FDA definition: Two products are considered to be bioequivalent when they are equal in the rate and extent to which the active pharmaceutical ingredient (API) becomes available at the site(s) of drug action.
-
Where are Bioequivalence Studies used?
A Bioequivalence study can be used to prove a generic product is bioequivalent to the already marketed reference product, and the Bioequivalence study will be part of the abbreviated New Drug Application (ANDA). A generic drug contains the same active ingredient as the reference product. The generic product to be marketed will have to meet the regulatory agency requirements for Bioequivalence clinical trial since the bioequivalent products to be expected the similar clinical effects based on the equivalent drug concentration.
A Bioequivalence study can also be part of the New Drug Application (NDA) when the different The same bioequivalence principles apply to new drugs when different formulations of an active ingredient are compared. It can also be used to support the different formulation changes for one kind of NDA submission called 505 (b)(2). Bioequivalence and bioavailability studies are essential in early (Pilot Bioequivalence) and late (Pivotal Bioequivalence) clinical development of drug candidates.
-
Why are Bioequivalence Studies important?
Only products that have been proven to be bioequivalent should be used interchangeably. On scientific grounds, there is no reason to be concerned about substituting a generic product for a branded product that is confirmed as being bioequivalent.
-
How do you measure Bioequivalence?
Bioequivalence is determined by comparing the pharmacokinetics of two products (test and reference), and based on the relative bioavailability of the innovator medicine versus the generic medicine. It is measured by comparing the ratio of the pharmacokinetic variables for the innovator versus the generic medicine, where equality is 1. BE is to be determined by comparing the peak plasma concentration (Cmax), time to achieve a maximal concentration (Tmax) and the extent of absorption (area under the concentration-time curve, AUC) of the products.
-
How to design a Bioequivalence study?
In a standard bioequivalence trial, both test and reference products are administered on separate occasions to healthy volunteers with specific washout period. The bioequivalence trial usually utilizes normal, healthy volunteers. The study is mostly crossover design in which each subject is assigned by a randomization number with the order (sequence) of the test or reference drug administration separated by a sufficient washout time.
