Adaptive Sample Size Sequential Design in Bioequivalence Trials
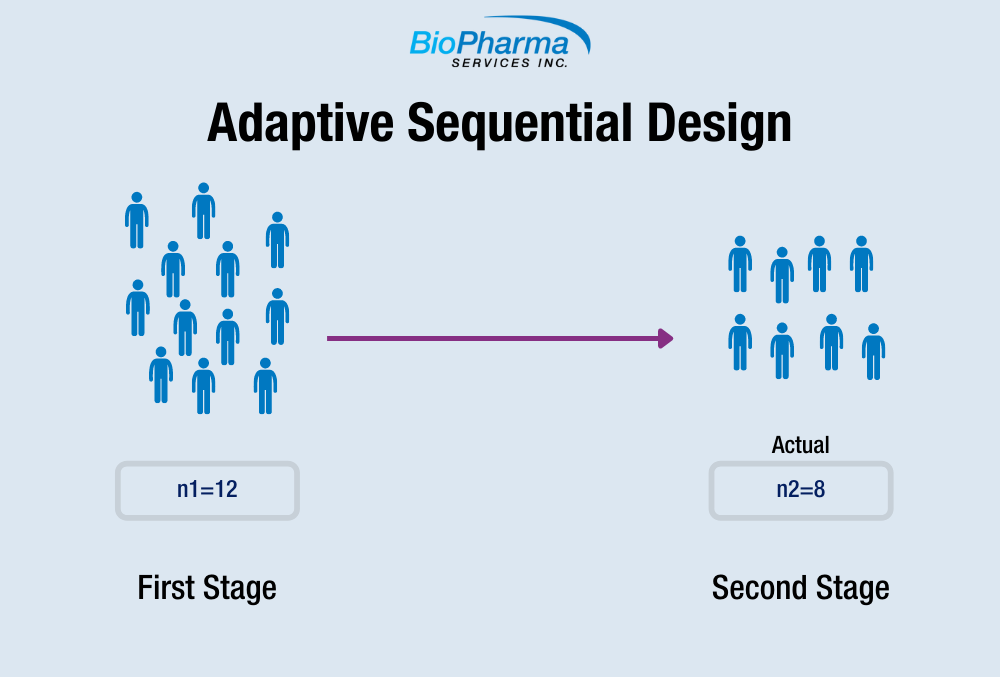
What is the Adaptive Sample Size Sequential Design?
Adaptive Sequential Designs have been widely used in human clinical trials for new drug development since the 1970s, and can provide a variety of advantages over non-adaptive designs. In 2008, Potvin et al. proposed a hybrid of a group sequential design and adaptive sequential design: a two-stage group sequential design with sample size re-estimation based on the variance estimated from the first stage.[i]
In Bioequivalence trials, this design has been accepted and recommended by the major regulatory bodies (US FDA, EU/EMA, Health Canada). Potvin recommended three methods: methods B, C, and D, which were three variations of adaptive sequential methods based on type I error level, power calculation, stopping rules, and re-estimate sample size.[i]
Major Advantages of Adaptive Sequential Designs
There are a number of major advantages when discussing adaptive sequential design;
- Statistical efficiency: an adaptive design can provide a greater chance to detect a true drug effect (i.e., greater statistical power) than a comparable non-adaptive design.
- Ethical considerations: an adaptive design can stop a trial early if it becomes clear that the trial is unlikely to demonstrate that effectiveness can reduce the number of patients exposed to the unnecessary risk of an ineffective investigational treatment.
- Dynamic understanding of drug effects.
- Acceptability to stakeholders: the sponsors might be more willing to commit to a trial that allows planned design modifications based on accumulating information because of the added trial flexibility.
- Multiple Trials: adaptive design provides the chance to avoid a trial with inadequate statistical power, and therefore helps ensure that the trial would efficiently and reliably achieve its objective.
- Unprecedented COVID-19 challenges: study interruptions that can lead to using multiple batches in a single study due to batch expiration, partial data due to participant drop out.
- The variability of uncertainty: there is very little information on the intra‐subject variance, or the estimate of the intra‐subject variance from the literature has large uncertainty.
- The assessment of safety and effectiveness: the trial was to select an appropriate dose and confirm the safety and effectiveness of that dose in a timely manner.
Key Summary of Research History:
In the late 1970s, Pocock, O’Brien, and Fleming proposed group sequential design tests that allow for one or more prospectively planned interim analyses of comparative data with prespecified criteria for stopping the trial.[ii] A variety of methods exist to determine appropriate stopping boundaries for the interim and final analyses, such that the Type I error probability is appropriately controlled. For example, the O’Brien-Fleming approach tends to require very persuasive early results to stop the trial for efficacy.[ii]
Alternative approaches such as that proposed by Pocock require less persuasive early results and have higher probabilities of early stopping. These and other approaches rely on prospective planning of both the number of interim analyses and the specific sample size or number of event targets at which those analyses will occur.
The Lan-DeMets alpha-spending approach accommodates varying levels of evidence for early stopping by specifying a function for how the Type I error probability is spent throughout the trial, while also allowing for flexibility in determining the number and timing of interim analyses. The possibilities of stopping a study earlier or adjusting the sample size during the conduct of a study are attractive properties of these designs.
What is the Current Thinking From the Regulatory Bodies?
The adaptive sequential designs to a clinical trial design, accepted by FDA, EMA and Health Canada, are widely used in innovative research due to significantly higher savings and no unnecessary exposure of people to drugs. The adaptive sample size sequential approach is convenient for bioequivalence study designs. The acceptable approach varies with the different regulatory bodies. For example,
- EMA recommends method B (Refer to GUIDELINE ON THE INVESTIGATION OF BIOEQUIVALENCE).
- Health Canada also accepts group sequential design and method C (Refer to Conduct and Analysis of Comparative Bioavailability Studies)
- FDA can accept method C when the drug CV is high.
What are Methods B and C?
Method B
Method B specifies the same value for α (=0.0294) at each stage. The Bioequivalence is evaluated after Stage 1 using an α level of 0.0294. If Bioequivalence is demonstrated, the study will stop. If Bioequivalence is not demonstrated, the statistical power will be evaluated using the same α level of 0.0294 at stage 1. If the power is ≥ 80%, the study will stop and Bioequivalence would not be concluded. If the power is < 80%, the study can continue onto Stage 2. The sample size for Stage 2 is estimated based on the intra-subject variability calculated from the variance at Stage 1 and a Test/Reference ratio of 0.95 and a pre-defined α level of 0.0294. BE is evaluated at Stage 2 using data from both Stage 1 and Stage 2 using an α level of 0.0294. The study stops regardless of whether Bioequivalence is demonstrated or not.
Method C
Method C evaluates the power at Stage 1 using an α level of 0.05. If the power is greater than or equal to 80%, evaluate Bioequivalence at stage 1 using an α level of 0.05 and stop whether Bioequivalence is met or not. If the power is less than 80%, Bioequivalence is evaluated at Stage 1 using an α level of 0.0294. If Bioequivalence is demonstrated, the study stops. If BE is not demonstrated, the study can continue onto Stage 2. The sample size for Stage 2 is estimated using the variance at Stage 1 and an α level of 0.0294. The Bioequivalence is evaluated at Stage 2 using data from both stages (α=0.0294). The study stops regardless of whether it has been demonstrated or not.
When do the Methods B and C will be Used
Both of the methods can be used based on the intended submission when running into the following challenges during the planning of a trial.
- An unprecedented pandemic led to the higher drop-out rate due to withdraw and dismiss
- The batch expired due to unexpected recruitment challenges;
- There is little information on intra-subject variability;
- The estimate of the intra‐subject variance from the literature has large uncertainty;
- Highly variable drug or highly variable drug product;
Biopharma Services is Your Partner for the Application of Adaptive Sample Size Sequential Design in Generic Drugs.
The use of this design in the bioequivalence study seems to be a beneficial alternative to the 2 × 2 crossover study in some situations. Statistical methodology to assess bioequivalence is sufficiently developed and described. In the past, BioPharma Services has successfully conducted the adaptive sequential design trials and helped the clients to get approval from the regulatory body on some products. We are confident that the statistical capacity, hands-on experience, and expertise will provide the best strategies for you and assist you to make your product on the market efficiently.
Find out why BioPharma might be the right partner for you! Learn more about BioPharma and the wide array of bioanalytical services we provide.
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials 1/2a and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.