PET Imaging in CNS First-in-Human Clinical Trials
Central Nervous System (CNS) disorders, including neurological and mental diseases, are among the most important causes of disability worldwide.
Positron Emission Tomography (PET) imaging has emerged as an essential tool in the process of drug development for CNS disorders, enabling the investigation of molecules within the brain.
Drug Development for CNS Disorders
For many CNS disorders, the currently available therapeutic approaches have limited efficacy and, in some cases, may provide only symptomatic relief rather than disease-modifying effects. Therefore, an urgent need exists to develop novel and improved therapeutics for neurological and mental disorders. However, drug development for CNS disorders is associated with significant challenges, including:
- The presence of a blood-brain barrier, limiting the access of molecules to the brain
- The complex pathophysiology of CNS disorders
- The poor predictive value of preclinical models
- The scarcity of biomarkers for target engagement
- Challenges associated with volunteer recruitment
The Emerging Role of PET Imaging in
Clinical Neuroscience Research
Molecular Imaging, and especially PET imaging, has created new opportunities for advancing CNS drug development by enabling the investigation of biological processes in the brains of living organisms without the need for invasive procedures like biopsies. PET scans are based on injecting a minute amount of a radioactive ligand (radiotracer) intravenously. This is followed by detecting signals from the radiotracer, reconstruction of 3D images, and observation of biological processes in living organisms.
PET imaging can be used to monitor cellular metabolism. However, it can also focus on specific targets, which in the CNS include monitoring synaptic neurotransmitter receptors, neurotransmitter transporters, or abnormal deposits of proteins, and can provide information regarding neuronal plasticity. Moreover, since PET radionuclides have low steric hindrance, they can label small molecules that can cross the blood-brain barrier while preserving their pharmacological activity.
PET neuroimaging is emerging as a valuable therapeutic assistance tool that can help screen patients’ eligibility for treatments and monitor therapeutic effects. PET scans can also be combined with anatomical imaging modalities, including computed tomography (CT) or magnetic resonance imaging (MRI), providing synergistic functional and anatomical information. However, the use of PET imaging in clinical trials is also associated with significant difficulties.
PET Imaging Challenges in CNS First-in-Human Clinical Trials
Clinical & PET Imaging Site Coordination
Patient Recruitment & Scheduling
Source Document Management
Expert
Staff
Delegation
Regulatory
Study
Aspects
Working with an experienced full-service CRO like BioPharma Services can guarantee the competent and efficient design of clinical trials incorporating PET studies.
PET Imaging in CNS Clinical Trials
& Clinical Practice
Advances in PET imaging have led to its increased implementation in CNS FIH clinical trials. One of the key areas of PET scans’ use for the analysis of CNS drug candidates is to confirm target engagement, establish pharmacodynamic-pharmacokinetic relationships, and define the doses of study drugs.
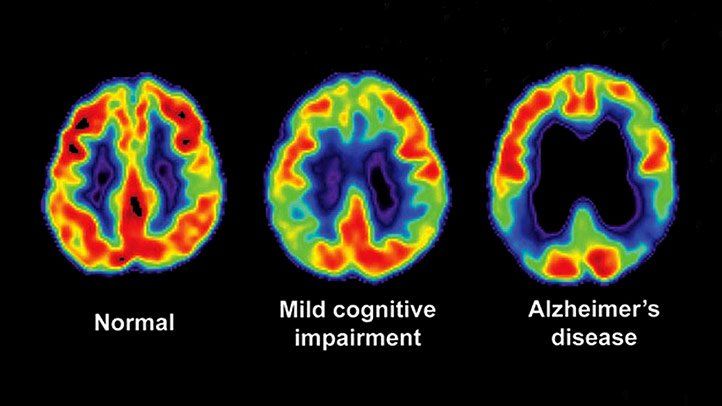
PET imaging has been utilized to study the extent of neuroinflammation in disorders affecting the CNS, such as multiple sclerosis and amyotrophic lateral sclerosis (ALS), as well as to observe the impact of therapeutic drug candidates on neuroinflammation. In addition, PET imaging has gained significance in the research on neurodegenerative disorders, because it can track amyloid and tau deposits in patients affected by Alzheimer’s disorder and a-synuclein in patients with Parkinson’s disease and the effect of drug candidates on them.
PET imaging may help assess patients’ eligibility for inclusion in FIH clinical trials or evaluate the effects of drug candidates. PET neuroimaging may aid the development of biomarkers for early clinical diagnosis or detection of preclinical disease, including an FDA-approved imaging agent (florbetapir F18) that detects beta-amyloid plaques in the brain. Florbetapir F18 has been implemented in the diagnostic assessment of individuals affected by dementia or cognitive impairment. A negative florbetapir F18 scan is considered inconsistent with a neuropathological diagnosis of Alzheimer’s disease at the time of imaging.
Ready To Get Started?
Complete the Form below to schedule a Discovery Call with a member of our medical team to learn how BioPharma Services can be your trusted clinical trial partner!
Schedule a Discovery Call
You can unsubscribe at any time. For more details, please read our Privacy Policy.
