Clinical Trial Data Management Services
Clinical Data Management is an essential clinical research process involving collecting, processing, storing, and managing clinical trial data. Its purpose is to ensure that the collected data are high quality, accurate, complete, consistent, and compliant with regulatory requirements.
Significance of Clinical Data Management Services for Drug Development Programs
The successful completion of clinical trials requires their thoughtful design and conduct and the generation of a comprehensive clinical data management strategy. Such strategies help ensure that the acquired clinical trial data are of high quality, comply with regulatory requirements, and can be used to make informed decisions regarding the safety and efficacy of a new chemical entity (NCE) or a new investigational medicinal product (IMP). In addition, effective clinical trial data management may streamline the drug development process, potentially shortening the timeline for developing NCEs and IMPs.
BioPharma Services, Inc. (BioPharma) is a full-service clinical research organization (CRO) that offers innovative and all-encompassing solutions for early-phase clinical trials. Our multidisciplinary, award-winning team includes competent and experienced data management scientists who work individually with our clients to develop comprehensive data management strategies for their clinical drug development programs. Collection to data review, query management and mapping under Study Data Tabulation Model (SDTM) standards and CDISC conversion, our goal with our clinical trial services is to hand over high quality study data to our clients in the most expeditious manner. Learn more about our team.

Clinical Trial Data Management: A Complex and Multifaceted Process
The process of clinical trial data management is complex. It requires the input of a team of individuals with complementary competencies, including data managers, clinical data coordinators, database programmers/designers, data entry associates, medical coders, and quality control associates.
Clinical trial data management includes several phases.
Database Design
Databases are software applications that are designed to facilitate clinical data management and can be used for multiple studies. Before their implementation, they undergo a system validation that ensures data security and compliance with user and regulatory requirements.
Data Collection
The goal of data collection is to acquire data that are accurate, of high quality, complete, consistent, and compliant with regulatory requirements. In clinical trials, study participants’ data can be collected using various methods, including electronic data capture (EDC) systems or paper-based forms.
Data Processing & Transformation
Next, the collected data are entered; checked for completeness, accuracy, and consistency; cleaned; and validated. A systematic approach should be utilized to check for missing data and data errors, and automated algorithms for the detection and handling of such data have been developed to streamline the process. In addition, some of the collected data may not be in a format compatible with the software used for statistical analysis. Such data may need to be recategorized and recoded, and new variables may be derived in accordance with the analytical plan of the clinical trial.
Data Analysis, Storage, & Privacy
In the final phase, the cleaned and validated data are used for statistical analysis, which can provide data on the safety and efficacy of an NCE or IMP, and the obtained results can be presented as tables and figures. Moreover, as the safety and rights of study participants should always be protected, data privacy should be an utmost priority for data management teams.
BioPharma Services’ Clinical Trial Data Management Team
Our expert clinical trial data management team is involved in all clinical trial stages, from its design to data archival, to ensure that data are appropriately collected, processed, stored, and analyzed. The clinical data management services offered by BioPharma are tailored to the individual needs of our clients and offer them comprehensive and efficient data management solutions:
A Competent & Dedicated Data Management Team
BioPharma’s data management team includes professionals with extensive experience handling data from early-phase clinical trials, bioequivalence studies, and human abuse potential studies. The expertise of our team is underscored by the successful completion of over 2,000 clinical trials. Moreover, our data management experts are committed to maintaining up-to-date competencies by participating in continuing education activities.
Close Collaboration with Other Experts Within Our Organization
BioPharma Services’ data management team cooperates closely with other experts in the organization, including biostatisticians, clinical pharmacologists, safety physicians, bioanalytical scientists, and regulatory scientists. In this context, the interactions of our data managers with our highly qualified team of biostatisticians and programmers, are especially beneficial. The synergistic collaborations among the members of our organization enable us to comprehensively address the needs of our clients’ clinical drug development programs.
Innovative & Creative Data Management Solutions
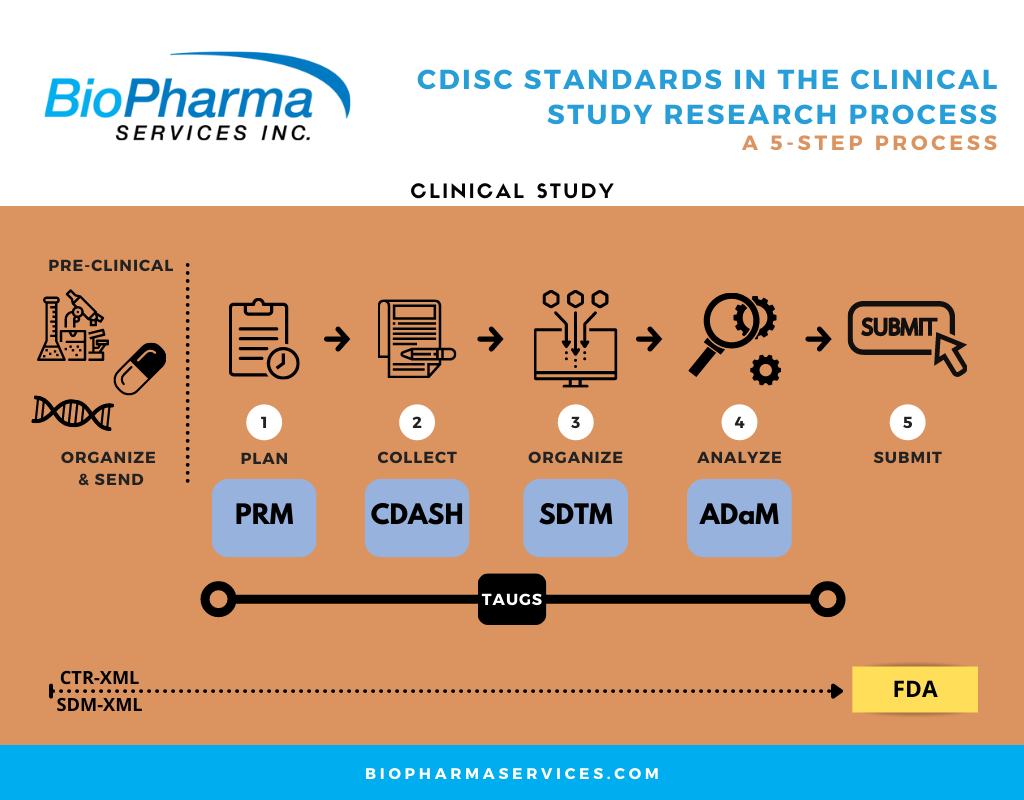
BioPharma‘s clinical trial data management team is constantly designing innovative and creative solutions to expand and improve our clinical trial services’ processes and procedures that will allow us to exceed client expectations. We understand clinical trial data management is much more than simply collecting data electronically. From data collection to data review, query management, and mapping under Study Data Tabulation Model (SDTM) standards and CDISC conversion, our goal is to hand over high-quality study data to our clients in the most expeditious manner.
Comprehensive Clinical Trial Data Management Solutions
Our team supports all aspects of clinical trial data management, including designing an efficient clinical data management plan, implementing procedures ensuring high-quality data acquisition, and using appropriate monitoring services. BioPharma Services has extensive experience with early-phase clinical trials, including challenging study designs and routes of administration, as well as niche expertise in human abuse potential studies.
Compliance of Clinical Trial Data Management With Regulatory Requirements
The clinical trial data management process should comply with strict regulatory requirements set out by both local (depending on the regulatory region) authorities and international standards. Thus, one of the critical obligations of data management teams is to ensure that the acquired data and procedures align with the requirements of the relevant regulatory agencies. In addition, the Clinical Data Interchange Standards Consortium (CDISC) has set out data standards, and converting clinical and metadata to comply with them streamlines the regulatory review and approval process.
- BioPharma’s CDISC Data Conversion Services – We competently and efficiently support our clients with dataset mapping according to SDTM standards and CDISC conversion. Our team has expertise in creating SDTM annotated case report forms (CRFs), SDTM datasets, pooled analysis datasets (ISS/ISE), ADaM analysis datasets, and data handling reports.
- BioPharma’s experience working with regulatory agencies – Our team has worked with numerous regulatory agencies, including the United States Food and Drug Administration (FDA), Health Canada, European Medicines Agency (EMA), United Kingdom MHRA, and ANVISA. It has undergone over 30 successful regulatory inspections. BioPharma’s data management experts draw on the regulatory expertise of our organization to ensure that all aspects of clinical trial data management comply with the relevant regulatory requirements.
BioPharma’s Extensive CDISC Data Conversion Services include:
-
- SDTM annotated CRFs
- SDTM datasets
- ADaM analysis datasets
- Pooled analysis datasets (ISS/ISE)
- Define.xml metadata and Define.pdf
- CDISC controlled terminology
- Data handling reports
- FDA reviewers’ guide
- Electronic common technical document (eCTD) structure
BioPharma’s Clinical Data Management Services include:
-
- Data management plan (DMP) development
- Electronic case report form (eCRF) design and development
- Database build & design
- Data validation specifications
- Clinical trial data management
- Edit check programming & testing
- Collection and review of pharmacodynamic data
- Single or double data entry
- EDC training
- eCRF completion guidelines
- Query management
- Third-party data integration
- Customized clinical data management programming
- Safety data management & reconciliation
- Data export/transfer
- Interim analysis readiness
- Medical coding (CM, AE, MH)
- Serious adverse event (SAE) reconciliation
- Customized status reporting and data listings
- Database closeout and delivery
Schedule a Discovery Call
You can unsubscribe at any time. For more details, please read our Privacy Policy.
Data Management FAQ
-
What is Clinical Data Management and Who is Responsible?
Clinical data management (CDM) is a critical process in clinical research that involves the collection, processing, storage, and management of data obtained during a clinical trial. The purpose of CDM is to ensure that the data collected is of high quality, accurate, complete, and consistent, and that it meets regulatory requirements. CDM plays a crucial role in ensuring the integrity of clinical trial data, which is essential for making informed decisions about the safety and efficacy of a drug or medical device. The drug development sponsor of a clinical trial is responsible for ensuring that data management activities are conducted in compliance with regulatory requirements and industry best practices.
-
What are the Different Types of Data Management Systems in Clinical Trials?
There are several types of CDMs used in clinical trials, including:
- Electronic data capture (EDC) systems: These are software applications that enable researchers to collect and manage clinical trial data electronically, rather than on paper. They provide tools for data entry, data validation, and data query management.
- Clinical data warehouses (CDWs): These are centralized data repositories that store data from multiple clinical trials, allowing researchers to analyze data from different studies.
- Clinical trial management systems (CTMS): These are software applications that provide tools for managing various aspects of a clinical trial, including data management.
- Randomization and trial supply management (RTSM) systems: These are software applications that enable researchers to manage the randomization of study participants and the distribution of study medications.
-
What are the Roles and Responsibilities of the Data Management Team in Clinical Trials?
The data management team in a clinical trial is responsible for managing the entire data lifecycle, from the collection of data to its archiving after the study is completed. The key roles and responsibilities of the data management team include:
- Developing data management plans (DMPs) that outline the procedures for data collection, quality control, and data cleaning.
- Developing and validating data capture forms and electronic data capture (EDC) systems.
- Managing the data collection process, including data entry, verification, and query resolution.
- Conducting data quality control checks to ensure the accuracy, completeness, and consistency of the data.
- Creating and maintaining data management documentation, including the data management plan, data validation plan, and data cleaning plan.
- Preparing data for statistical analysis and creating data listings
- Archiving study data and documentation in accordance with regulatory requirements.
-
What are the Three Phases of Clinical Data Management?
The three phases of clinical data management are:
- Data collection: This phase involves the collection of data from study participants using a variety of methods, including paper-based forms or electronic data capture (EDC) systems.
- Data processing: In this phase, the collected data is processed and checked for completeness, accuracy, and consistency. This involves data entry, data cleaning, and data validation activities.
- Data analysis: In the final phase, the cleaned and validated data is used for statistical analysis to determine the safety and efficacy of the investigational drug or medical device. The data is analyzed using statistical software, and the results are presented in the form of tables, listings, and figures
-
What Regulations and Standards do CROs Follow for Clinical Data Management?
The following are some of the regulations and standards that CROs such as BioPharma Services must follow for clinical trial data management:
- USA
- FDA Regulations: The Food and Drug Administration (FDA) regulates clinical trials in the United States, and CROs must comply with its regulations for data management. FDA regulations include the Code of Federal Regulations (CFR), which outlines the standards for data management, including data collection, storage, and analysis.
- Health Insurance Portability and Accountability Act (HIPAA): The HIPAA Privacy Rule protects the privacy of patient health information and sets standards for data management, storage, and transmission. CROs must follow HIPAA regulations to ensure that patient data is protected and kept confidential.
- CANADA
- Health Canada: High focus on the quality and efficacy of pharmaceutical products and clinical trials. Compliance with Good Clinical Practice (GCP) principles, both at the international level and the Canadian guideline for GCP, ensures ethical and scientifically sound data management.
- Personal Information Protection and Electronic Documents Act (PIPEDA). PIPEDA is a federal privacy law that governs the collection, use, and disclosure of personal information by private sector organizations in Canada.
- International Standards
- International Conference on Harmonisation (ICH) Guidelines: The ICH has developed several guidelines for clinical trial data management that CROs must follow, including ICH GCP (Good Clinical Practice) guidelines. These guidelines provide a framework for ensuring that clinical trials are conducted ethically, and that data is collected, analyzed, and reported accurately.
- Electronic Records and Electronic Signatures (21 CFR Part 11): 21 CFR Part 11 provides guidelines for electronic records and signatures used in clinical trials. CROs must follow these guidelines to ensure that electronic records are accurate, secure, and reliable.
- USA
