Oncology Drug Development Formulation in Clinical Research Trials
BPSI’s success story using normal healthy volunteers for Cost-efficient clinical development of a generic or improved formulation of oncology drug(s)

Introduction
Growing concerns about the high number of cancer-related mortality have increased the demand for oncology drugs. However, these oncology drugs are generally pricey and unaffordable for the general population, which makes the market for these drugs exclusive with limited accessibility and hinders the treatment of cancer patients. This is where generic oncology drugs (copy of a brand name product) or new drug applications (505 (b)(2)) (improved or altered version or new use of a previously FDA-approved drug) come into play. These classes of drugs save 12 to 15 years from discovery to commercialization.
More importantly, generic oncology drugs cost around 80% less than their branded oncology treatments, while also providing good treatment efficacy. According to the market research report from FMI, the worldwide generic oncology market is expected to grow at a remarkable Compound Annual Growth Rate (CAGR) of 6% through 20283. One factor that allows to reduce the cost of development, and maximize manufacturer profits while maintaining an affordable price of novel and generic oncology drugs, is the use of healthy volunteers (HVs) in clinical trials.
In this article, we focus on the role of HVs in clinical trials for antineoplastic agents and the successful strategies that BioPharma Services Inc. (BPSI) has used in this setting.
Dosing on HVs vs Patients
In oncology drug development, the early pharmacokinetic (PK) and safety endpoint studies (Phase 1 Clinical Trials and comparative bioequivalence and bioavailability studies may enroll HVs instead of the patients. Although the use of HVs in those studies may raise some concerns, mainly over subjects’ safety, numerous factors and incentives support the use of HVs in oncology trials. Studies in HVs allow rapid enrollment, lower dropout rates, rare confounding by comorbidities and/or concomitant medications, and better participant compliance leading to fewer protocol deviations, and more reliable data. The relationship between adverse events and study drugs can be easily identified.
Furthermore, enrolling HVs not only increases study accrual rates for single- and multiple-dose PK endpoint-driven studies but also eliminates the ethical concerns of enrolling patients with advanced disease in a short-term study at subtherapeutic doses (e.g., Phase 1 Clinical Trials study collect preliminary safety data)4. To note, evaluating the decision to include HVs for oncology drugs in BA/BE studies is based on the safety evaluation of the recommended study dose when administered. For example, while Targretin® (Bexarotene) and Gleevec (Imatinib) can cause fetal abnormalities for long-term use, a single-dose BE study in a carefully screened population of healthy male subjects is unlikely to pose serious risks5.
How BioPharma Services Develops the Successful Way in Clinical Research Trials
Over the past years, BioPharma Services has conducted many successful BA and BE clinical trials of generic or improved formulations of oncology drugs, and table 1 summarizes our experiences.
| Product name | Target/Mechanism of Action | Study design | Endpoints |
| Acalabrutinib | BTK inhibitor | Randomized, open-label, crossover | PK, Safety |
| Axitinib | Tyrosine kinase inhibitor | Randomized, open-label, crossover | PK, Safety |
| Cladribine | Interfere with DNA synthesis | Randomized, double-blind, parallel, placebo-controlled | PK, Safety, tolerability |
| Dasatinib | Tyrosine kinase inhibitor | Randomized, open-label, crossover | PK, Safety |
| Erlotinib | EGFR inhibitor | Randomized, open-label, crossover | PK, Safety |
| Gefitinib | Tyrosine kinase inhibitor | Randomized, open-label, crossover | PK, Safety |
| Ibrutinib | BTK inhibitor | Randomized, open-label, crossover | PK, Safety |
| Imatinib | Tyrosine kinase inhibitor | Randomized, open-label, crossover | PK, Safety |
| Lenvatinib | Tyrosine kinase inhibitor | Randomized, open-label, crossover | PK, Safety |
| Neratinib | EGFR inhibitor | Randomized, open-label, crossover | PK, Safety |
| Pazopanib | Tyrosine kinase inhibitor | Randomized, open-label, crossover | PK, Safety |
| Regorafenib | Tyrosine kinase inhibitor | Randomized, open-label, crossover | PK, Safety |
| Ruxolitinib | Janus kinase inhibitors | Randomized, open-label, crossover | PK, Safety |
| Sorafenib | Multi-kinase inhibitor | Randomized, open-label, crossover | PK, Safety |
| Sunitinib | Multi-kinase inhibitor | Randomized, open-label, crossover | PK, Safety |
| Tofacitinib | Janus kinase inhibitors | Randomized, open-label, crossover | PK, Safety |
Table 1. BioPharma Services clinical trials of oncology drugs in healthy volunteers
All the above clinical trials have undergone a thorough safety assessment and have had their study designs have been developed thoughtfully to consider volunteers’ safety. The following sections are the procedures implemented by BioPharma Services for clinical trials on oncology drugs and new investigational products.
a. Assessing the Safety Profile
Despite the discussed advantages, many scientists still hesitate to consider the HVs in a study involving anticancer drugs due to the high-risk profiles of cytotoxic chemotherapy. In recent years, oncology drug development has shifted from the exclusive use of cytotoxic agents to the addition or substitution of immunomodulatory and molecularly targeted agents with more acceptable safety profiles and lower risk of cytotoxicity. The safety profile of such non-cytotoxic compound(s) has made it possible to include HVs in oncology BA/BE and Phase 1 clinical trials.
How to Decide the Inclusion of Health Volunteers
At BioPharma Service’s inclusion of HVs is decided by talented BioPharma medical and scientific teams, the comprehensive assessment is illustrated in Figure 1. The final decision is based on the assessment of all the available nonclinical and clinical data at the time of proposing the trials in HVs, the nature of adverse events, and study design including appropriate inclusion/exclusion criteria and risk mitigation plan.
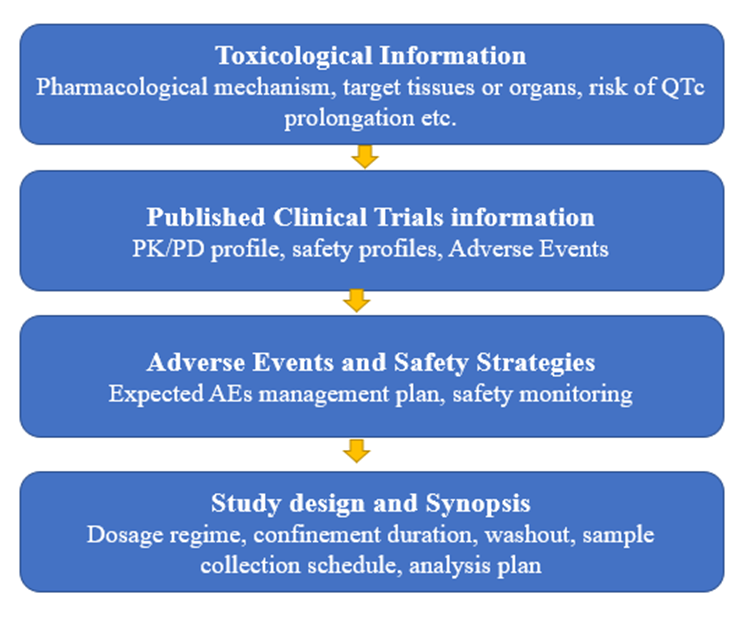
Figure 1. Feasibility assessment of inclusion HVs
Utilizing the Sentinel Approach as a Facilitating Tool
To mitigate risk in clinical trials of new investigational drugs, the “sentinel” approach is more often adopted for Phase 1 Clinical Trials. A typical sentinel group enrolled two to three subjects to assess the safety, tolerability, and/or pharmacology of the investigational dose before dosing the remaining subjects in the respective dose cohort. This approach is quite common for the first in human single- or multiple-ascending dose (SAD or MAD) trial, when the sentinel approach can be carried out for each dose level for an adequate period based on the anticipated AEs and mechanism of action of the drug candidate.
b. Assessing the Use of HV on a Case-By-Case Basis
At BioPharma Services, this approach is also recommended on a case-by-case basis for assessing the use of HV in BA and BE trials for 505(b)(2) and generic products if there is insufficient safety and tolerability data in healthy populations from the public domain accompanied within-house experience. Before the final decision is made to enroll HVs in BA and BE trial for either 505(b)(2) or generic submission purposes, a smaller scale SAD trial with a new formulation and placebo to be dosed with a sentinel approach. If the safety of subjects in this smaller scale SAD (often we can call it Pilot) is deemed to be acceptable and tolerated well by PI, a pivotal BA/BE trial will be designed with careful consideration of the information on safety and PK gained from the above SAD (pilot) study.
The pivotal BA and BE trial will be a crossover design to assess the relative bioavailability between the test and marketed product (RLD). The critical elements to consider for such a trial are the proper sample size to provide sufficient statistical power, proper blood sampling based on the pharmacokinetic profile, inclusion/exclusion criteria, safety measurement, and proper statistical method.
c. Successful Example – Using Scientific Justification And Choosing the Most Appropriate BA/BE Study Design
There is a lack of a standardized guide to determine if a new formulation or generic oncology drug is feasible to be conducted in HVs, many factors contribute to the decision on study site/country selection for this kind of clinical trial(s). Certain generic product-specific guidance released by regulatory agencies modified the study population according to newly available supportive safety evidence. This indicated that the agency may allow justification for the study population different from the guidance if the study design is sufficiently supported by literature data, medical and scientific justification, and an appropriate risk mitigation plan.
Take Imatinib as an example, according to FDA product-specific BE guidance, the subjects for this product should be patients. Based on published literature reviews, Imatinib was found to be relatively well tolerated. Moreover, EMA issued the BE guidance on Imatinib recommending healthy subjects as the study populations. Considering the available safety information and BioPharma Services in-house experience, we justified that the single oral dose of 400 mg of Imatinib in a typical crossover study dosed to HVs for the BE evaluation could be appropriate.
To date, we have conducted 11 successful clinical trials on this product and submitted to FDA, EMA, TPD, and TGA. The above example shows that new formulations and generic oncology drugs that have been systemically evaluated for safety can be used in clinical trials with HVs are well tolerated, the safety of the subjects is assured and the cost to the sponsor is significantly reduced.
How BioPharma Services can create the pathway of success for your oncology drug development project.
The clinical development of new formulations and generic oncology drugs is mostly challenged by the study population: HVs vs patients. BioPharma Services has conducted a series of clinical trials of such drugs in HVs and gained much success in this area through systematic safety assessments and thorough study designs, with a focus on minimizing risks to subjects. As a result, costs for drug developers for generic and 505 (b)(2) oncology products can be well controlled by collaborating with BioPharma Services.
Find out why BioPharma might be the right partner for you! Learn more about BioPharma Services and the wide array of bioanalytical services we provide.
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials 1/2a and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.



