State-of-the-Art Bioanalytical Testing
Bioanalysis is an indispensable component of clinical drug development, as it contributes to the pharmacokinetic and pharmacodynamic characterization of novel chemical entities (NCEs). The term bioanalysis encompasses identifying and quantifying different analytes in biological matrices.
Within clinical trials, the examined analytes include NCEs, their metabolites, and can also include potential biomarkers. The biological matrices analyzed in clinical drug development also vary and may consist of blood, plasma, serum, urine, tissue extracts, or cerebrospinal fluid.
The biopharmaceutical properties (such as solubility, permeability, or stability) or pharmacokinetic properties (such as volume of distribution, biological half-life, or clearance rate) of NCEs regulate the extent and rate at which NCEs reach their site of action.
Accordingly, poorly selected biopharmaceutical or pharmacokinetic characteristics are major factors contributing to the failure of clinical drug candidates. The competent and timely implementation of bioanalytical services in clinical trials contributes to the biopharmaceutical and pharmacokinetic characterization of NCEs and empowers timely decisions for clinical drug development.
Bioanalytical Assay Validation
The bioanalytical assays (a type of bioanalytical testing) used in clinical trials should be validated. Still, the specific validation requirements may vary depending on the trial’s phase and purpose (a concept known as fit-for-purpose validation). Clinical studies that will be included in filings to regulatory agencies such as investigational new drug applications (INDs), new drug applications (NDAs), or abbreviated new drug applications (ANDAs) to the United States Food and Drug Administration (FDA). The studies require the use of assays with the highest degree of validation. However, exploratory studies may require a lower degree of validation.
Bioanalytical assays should be validated with respect to their analytical and clinical aspects. Analytical assay validation is the most important component of the assay validation process. As a part of it, the assay’s sensitivity, specificity, accuracy, precision, and quantification range should be determined and characterized.
In addition, the effects of dilution, the stability and recovery of the analyte, and the performance of critical reagents, reference standards, and quality controls should be evaluated carefully. After the analytical validation is completed, the relevance of the assay for the clinical outcome of interest and its clinical utility should be assessed.
Steps Involved in Bioanalytical Analysis
Bioanalytical testing involves multiple steps including sample collection, processing, storage and extraction, as well as analyte detection and quantitation.
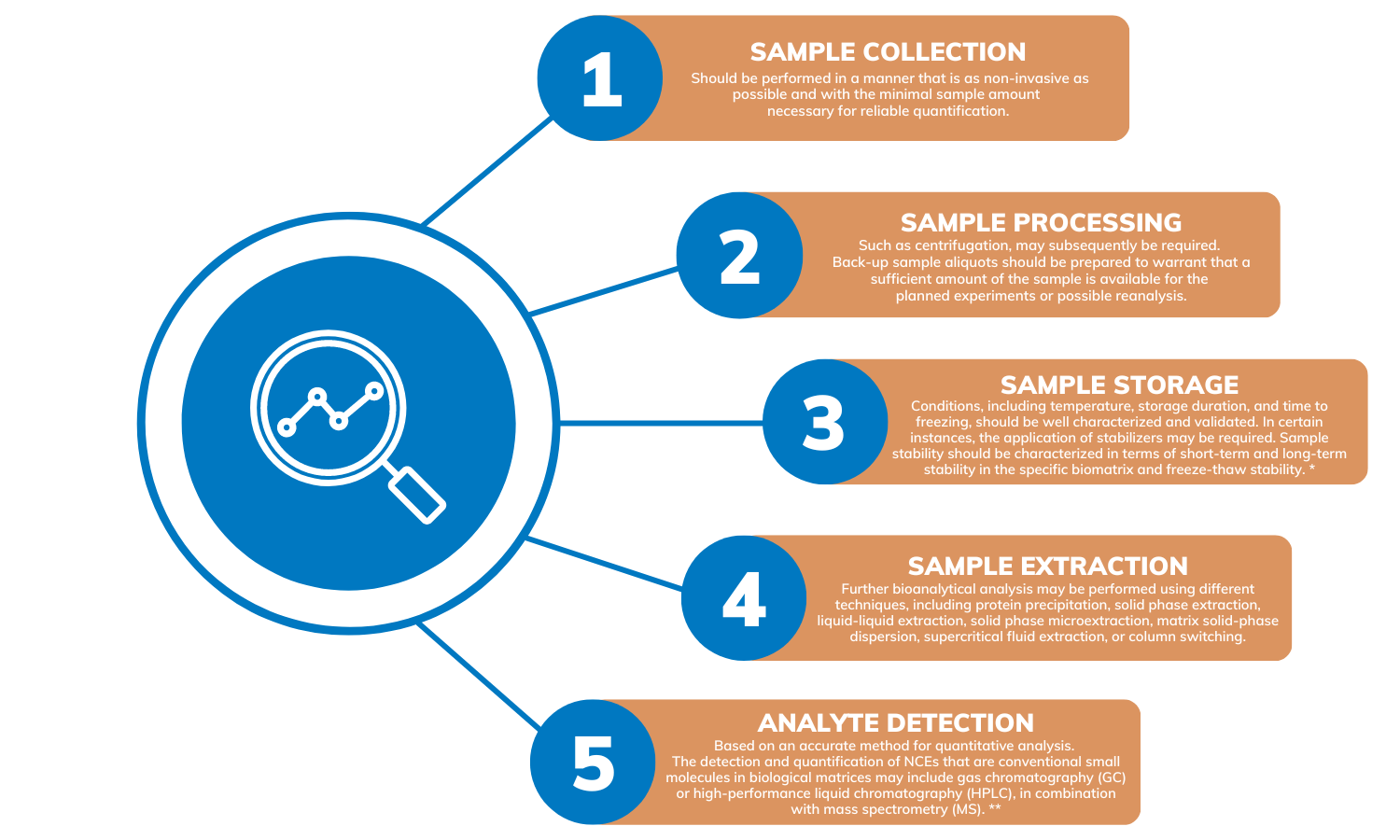
* The back-up capacity and the alarm system of the freezers storing the samples should be maintained. In addition, all associated documentation that efficiently records and tracks the sample collection, storage, and stability should be preserved.
** The most widely accepted method is probably HPLC coupled with tandem MS (HPLC-MS/MS). Other methods may include HPLC coupled with MS (HPLC-MS), GS coupled with MS (GC-MS), or GC coupled with tandem MS (GC-MS/MS).
Bioanalytical Testing in Early-Phase Clinical Trials
During early-phase clinical trials, the pharmacokinetic disposition of an NCE is evaluated most frequently in normal human volunteers (NHVs). Bioanalytical testing in this phase may include an evaluation of single or multiple doses, an assessment of the relative bioavailability of different formulations, and an evaluation of a food effect potential. The characteristics of early-phase clinical trials pose special requirements to bioanalytical assays used during this stage of drug development.
Developing Robust Bioanalytical Assays for Sponsors
The robustness of the bioanalytical assays used in clinical trials should be verified to empower reliable data collection.
Sufficient Sample Stability
A demonstration of sample stability is a critical component of bioanalytical method validation. Sample stability (most commonly under frozen conditions) for a sufficiently long period of time is required for bioanalytical testing included in early-phase clinical trials. The stored samples may be used as back-up samples or for potential reanalysis.
Optimizing Assay Turnaround & Throughput
Designing assays with rapid turnaround times and high throughput would facilitate timely decision-making regarding dose escalation.
Ensuring Assay Suitability Quantification Range
It should be confirmed that the sensitivity range of the assay is relevant to the NCE concentration expected to be achieved in the trial or to the expected concentrations of potential metabolites.
BioPharma Services, Inc. offers comprehensive bioanalytical services in the context of early-phase clinical trials. As a full-service contract research organization (CRO), we readily integrate the acquired bioanalytical data in the design and conduct of early-phase clinical trials.
A Wide Range of Validated Bioassays
BioPharma Services has developed and fully validated over 230 bioanalytical assays to support the diverse bioanalytical needs of our client clinical trial programs. The validated bioassays are intended for use in various matrices, including plasma, serum, whole blood, or urine. Moreover, we continuously develop and validate novel assays. Importantly, we can also develop custom methods of bioanalytical testing to address the specific needs of our clients’ bioanalytical studies.
Proficient Bioanalytical Experts
BioPharma Services has established a team of bioanalytical experts that includes 25 bioanalytical scientists. The team is guided by Dr. Nicki Hughes, who has more than 25 years of experience in bioanalytical research. Our experts work on all aspects of bioanalytical research and development (R&D), assay validation, and subsequent assay production. They have consistently achieved higher-than-industry standards with an over 98% passing batch rate of the conducted bioanalytical studies.
A Boutique In-House Bioanalytical Laboratory
Assay development, validation, and analysis are carried out in BioPharma Services’ boutique, in-house bioanalytical laboratory services with state-of-the-art instrumentation. Here the team can assess samples from bioavailability, bioequivalence, and clinical pharmacokinetic studies. Our bioanalytical testing strategy is based on using liquid chromatography with tandem mass spectrometry (LC/MS/MS).
The in-house LC/MS/MS platforms includes five QTRAP 5500 systems and four API 4000 systems. With these platforms we can detect and quantify minute amounts of analytes in a variety of biological matrices with high sensitivity and specificity.
Subsequent data analysis relies on the use of Watson LIMS software, which provides in-depth insight into the findings of bioanalytical testing. The quality of bioanalytical testing conducted in BioPharma Services’ in-house laboratory has been acknowledged with a good laboratory practice (GLP) certification by the Standards Council of Canada (SCC).
A Wide Range of Bioanalytical Services
The bioanalytical services that BioPharma offers are multifaceted and cover the full range of bioanalytical testing involved in early-phase clinical trials.
-
-
Method Feasibility Studies.
We conduct method feasibility studies that critically analyze the potential use of novel bioanalytical assays.
-
Bioanalytical Assay Development and Validation.
Our experts also develop and validate novel bioanalytical assays tailored to the needs of our clients’ drug development programs.
-
Method Transfer.
In addition to developing novel assays, we can also transfer established bioanalytical methods for use in early-phase clinical trials.
-
Bioanalysis of Samples from Bioavailability, Bioequivalence, and Clinical Pharmacokinetic Studies.
The validated bioassays are routinely used for analytes from bioavailability, bioequivalence, and clinical pharmacokinetic studies.
-
Integration with the Expertise of a Full-Service CRO
Our bioanalytical services are fully integrated into the comprehensive solutions for early-phase clinical trials offered by BioPharma Services. The bioanalytical scientists, clinicians, and regulatory scientists in our team collaborate closely with each other. This multidisciplinary approach enables us to select the most suitable methods of bioanalytical testing for our clients’ early-phase clinical trials to facilitate clinical decision-making during the drug development process and to support regulatory filings.
Ready To Get Started?
Complete the Form below to schedule a Discovery Call with a member of our medical team to learn how BioPharma Services can be your trusted Bioanalytical partner!
Schedule a Discovery Call
You can unsubscribe at any time. For more details, please read our Privacy Policy.
