Why BioPharma
BioPharma Services strives for scientific excellence, outstanding quality, and inspired innovation. Discover the difference today.
The BioPharma Benefit
Why choose BioPharma Services for your next drug development program?
Let us show you The BioPharma Benefits of working with a niche CRO with massive impact.
Quality
From protocol development through to regulatory submission, quality is a given – every step of the way.
Responsive
Our dedicated Project Managers work closely with you to ensure all aspects of your trial are well managed with fully transparent communication.
Nimble
Working with BioPharma means you have a partner that is agile enough to pivot and make adjustments when needed.
On-Time Results
In-house experts including PK, Data Management and Medical Writing deliver the results you need, when you need them.
Here's Some Of
Our Achievements
 35+
35+
Regulatory Inspections
 2,200+
2,200+
Completed Trials
 20+
20+
Awards and Recognitions
Why BioPharma?
Since 2006, BioPharma Services has been committed to delivering excellence in early stage clinical research while delivering a best-in-class drug development experience to biotechnology and pharmaceutical companies worldwide.
We’re a Full-Service CRO with expertise in Phase 1 clinical trials, Bioequivalence & Bioavailability studies, Human Abuse Liability Studies and more for multiple therapeutic areas.
Working with BioPharma
Every drug development program is different, but the one that matters most is yours. We’re here to help you meet your goals for subject recruitment, bioanalysis and regulatory submissions with flexible, scalable solutions for all types of studies.
Learn more about how BioPharma can help you achieve your drug development objectives.

01
Contact BioPharma
Starting with your initial inquiry, a BioPharma Business Development Associate will review your clinical research objectives and needs and walk you through the next steps.

02
Work with Our Experts
Meet our clinical pharmacology team. After learning more about your drug, we will work with you to build a robust study design that meets your objectives for collecting and analyzing clinical evidence.

03
Dedicated BioPharma Study Team
After developing a project management plan that includes all deliverables and expectations, we assign a dedicated study team to manage your clinical research project from start to finish.

04
Learn More About the Benefits of Working with BioPharma
You have questions; we have answers. Contact us today to schedule a call with a Business Development Associate.
Our Capabilities
We provide a range of clinical research services for all stages of drug development, from study design to trial execution.
➞ Clinical Pharmacology studies in multiple therapeutic areas
➞ Complex studies that require niche medical expertise
➞ First-in-Human studies
➞ Pharmacokinetics studies
➞ Human abuse potential studies
➞ Bioequivalence studies
➞ Robust database of healthy volunteers for high throughput enrollment
➞ Innovative assays for bioanalysis of novel compounds
Choose an Industry leader for your Clinical Study!
State of the art facility. World class leaders. Innovative scientific expertise.
 |
Phase 1 & 2aBioPharma has created a Phase 1 Center of Excellence able to execute a wide variety of Phase 1 clinical trials for our global pharmaceutical community. With our Phase 1 center located in Toronto, Canada, BioPharma has been integral in the success of completing multiple types of Phase 1 trials for our valued clients. |
 |
Human Abuse PotentialBioPharma has amassed extensive experience in the conduct of clinical studies in opioids, narcotics and cannabinoid products. Under the expert care and guidance of our Principal Investigator, Dr. Isabella Szeto, we have created innovative and safe solutions to conduct studies such as propofol in healthy volunteers. |
 |
BioequivalenceBioPharma has built a brand of excellence in the conduct of Bioequivalence (BE) and bioavailability (BA) studies to support both generic and hybrid drug filings and 505(b)(2) New Drug Applications. Good Clinical Practice (GCP) principles, robust Quality Control processes and Quality Assurance expertise underpin our commitment to deliver excellence in clinical research while delighting our clients.
|
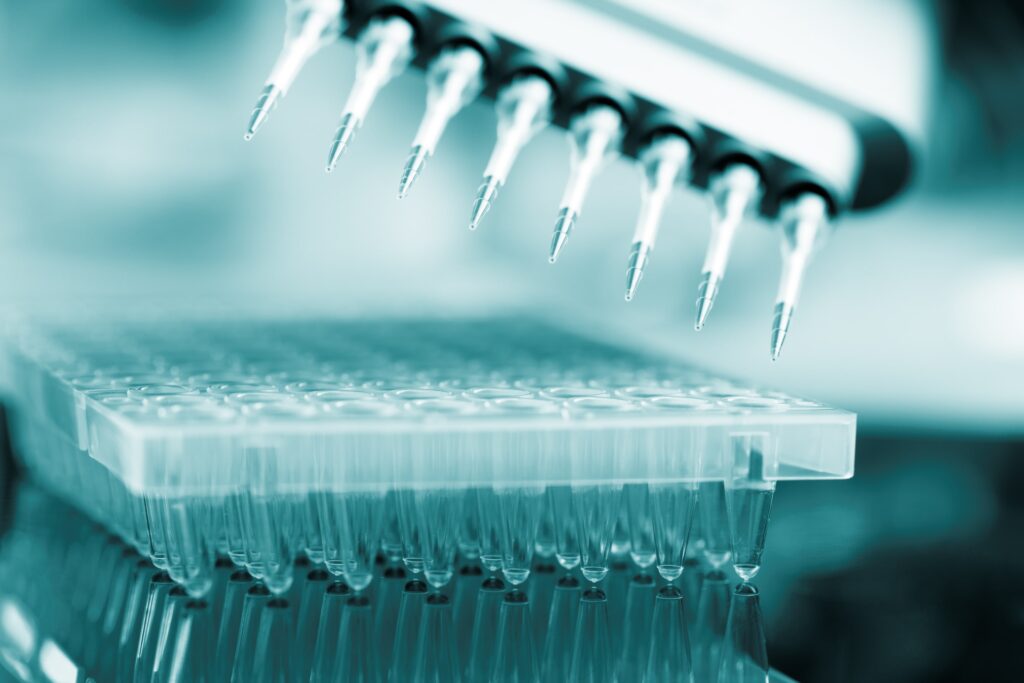 |
BioanalysisResearch & Development is one of our core strengths. Our Bioanalytical Lab, led by Dr. Nicki Hughes, a 20-year veteran in Bioanalytical Research, offers world class analysis with exceptional passing batch rates. |
 |
Scientific AffairsBioPharma’s in-house Scientific experts, including Pharmacokinetic and Clinical Research Scientists, work closely with clients on drug development programs for numerous therapeutic areas across multiple regulatory markets. With a blend of creativity and innovation, our scientists are skilled in the development of programs for all types of drugs from generic equivalents to novel chemical entities (NCEs). |
 |
PharmacokineticsBioPharma’s team of Pharmacokinetic Scientists have generated thousands of study designs to support drug submissions to regulatory markets across the globe. Backed by our own Medical Team, our Scientists employ the latest techniques to establish the appropriate route of administration, safe and effective therapeutic doses, and the frequency and duration of dosing to sustain the intended therapeutic effects. |
Book a discovery call to learn how BioPharma can help you reach your clinical research goals
The BioPharma Difference
World-class expertise and collaboration result in innovative solutions.

Our People
Two surgeons dedicated to advancing clinical science to improve patients’ lives founded BioPharma over 15 years ago.
Today, BioPharma is a well-established, mid-sized contract research organization operating two clinical research sites in Canada and the United States. Our team of more than 250 multidisciplinary experts works with clients like you with the same entrepreneurial spirit our founders demonstrated every day.
Our number one objective is to exceed your expectations through teamwork and our proactive, “can do” attitude.
Our Clinic
BioPharma’s 150-bed capacity at our paid research site in Toronto, Canada. The team at our research centre is unified with a common goal – to be a global leader in early-stage research, offering innovative, flexible and client-focused solutions to bring novel and affordable medicines to patients in need.
We are a clinical research organization led by a team of Medical Physicians, Scientists, and Clinicians. Clinical conduct and execution at BioPharma’s clinical facility are expertly tailored to meet the multi-faceted needs and requirements of the drug product, protocol design and study objective while ensuring the safety and well-being of our subject volunteers.


Our Lab
To ensure the successful application and reproducibility of an assay, one must first define the quality with which the assay was first developed.
The challenges of LC/MS/MS detection are compounded by the requirement for quantitation of low analyte concentrations, selectivity from endogenous compounds present in matrix while anticipating the metabolites that will be present in incurred samples. These complexities can all be managed with a rugged, robust and well developed assay that aligns with the regulatory requirements across multiple market jurisdictions.
R&D is the foothold of our lab that allows for the successful execution of the most complex of studies with timely and accurate results.
Schedule a Discovery Call
You can unsubscribe at any time. For more details, please read our Privacy Policy.

 35+
35+ 2,200+
2,200+ 20+
20+