Whole Blood Stability Determination: Overcoming Challenges of Drug Equilibration

Before a new chemical entity (NCE) or generic pharmaceutical product reaches the market, non-clinical (GLP) and clinical studies are required to demonstrate the safety and efficacy of the product. Pharmacokinetic profiling, which measures the concentration of the drug product in an appropriate matrix (e.g. plasma) versus time, as an important secondary endpoint of drug exposure. During a clinical study, healthy human volunteers will take the drug product as described under the approved study protocol.
Thereafter, blood from the subjects will be taken at specific time points and then be processed into the desired matrix type (e.g., plasma) for bioanalytical testing using a method validated to meet regulatory guidelines. While most of the validation experiment are conducted in the intended matrix (e.g., plasma), during the time before the whole blood is processed into plasma, the drug may be susceptible to further ex vivo degradation. To ensure accurate bioanalytical results, it is crucial to evaluate whole blood stability and to understand any potential degradation that may occur during this critical period.
Therefore, to ensure that the PK endpoint accurately reflects the level of the drug in vivo at the time of collection, there is a requirement for establishing the stability in whole blood during method validation to support the clinical sample processing, and we have presented other blogs describing the required addition of stabilizers to whole blood at the point of collection, (refer to our blogs about prodrugs, unstable metabolites, and sample stability).
Drug partitioning in whole blood fractions
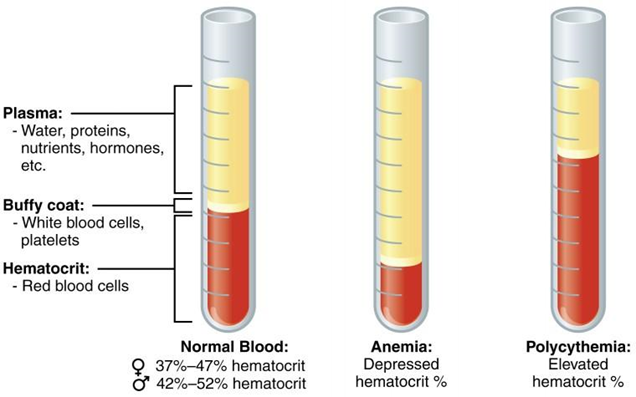
Before we start to describe the difficulties and barriers encountered when establishing whole blood stability in method validation, we must understand and consider the constituents in whole blood. There are four major components; plasma, platelets, red and white blood cells. Once the whole blood with anticoagulant has been centrifuged, it will be separated into plasma and hematocrit.
Many drugs preferentially partition into the aqueous plasma fraction in whole blood to bind to plasma proteins such as albumin, and for this reason plasma is most frequently selected as the matrix of choice for pharmacokinetic profiling. There are some highly lipophilic drugs however that can pass through the membrane of red blood cells (RBCs), which are the largest cell fraction in blood (size and number). RBSs may therefore serve as their natural compartment.
While bioanalysis in the RBC fraction alone has its challenges, in these instances whole blood rather than plasma is often selected as a preferred matrix. Tacrolimus is a clear example of this and as it is 99% associated with RBCs, whole blood is the preferred biological matrix in bioanalysis.
Consideration: Whole blood stability versus drug equilibration in blood fractions
During method development and validation of a bioanalytical method, a key factor to consider when determining the procedure to assess stability in whole blood is the tendency of the drug to partition between the red blood cells and the plasma proteins. As described above, this can be different for each drug moiety. The time needed to reach equilibrium between the red blood cells and plasma can vary in the in vitro system (spiking into whole blood) can take seconds up to hours. This process should be evaluated during method development and may require a customized experimental approach to better reflect the in vivo situation.
The time selected for whole blood stability assessment is typically representative of the situation during clinical samples processing. For most assays, where plasma is the selected final matrix, this is calculated from the time of the blood sample collected to its placement into the centrifuge to be processed into plasma, e.g., 2 hours.
Typically, whole blood stability is assessed by spiking blank freshly collected whole blood (equilibrated at 37 °C) with the analyte of interest. This in vitro samples which is mixed well then aged at room temperature, and/or under refrigerated conditions before being processed into plasma and analyte levels measured. Analyte stability in whole blood is considered acceptable when levels (concentration or peak area ratio) in the aged samples (2 hrs) is within ±1 5% of the levels at time zero (T0).
Examples where whole blood stability failed to meet acceptance criteria
|
Drug Name |
Percentage difference from T0 Formula : (area ratio Tx/area ratio T0) x 100% |
Reason of failure |
|
Amlodipine |
+18.9 % for LQC at 5 °C |
LQC >15% percentage difference for T2h versus To |
|
Celecoxib |
+28.3% for LQC at 5 °C +23.1% for HQC at 5 °C |
LQC and HQC >15% percentage difference for T2h versus To |
|
Vancomycin |
+17.5% for HQC T1h at 5 °C +20.4% for HQC T2h at 5 °C |
LQC and HQC >15% percentage difference for T2h versus To |
In each of these examples, an interesting pattern was observed, where for all the failures the aged samples (T1h & T2h) had higher drugs levels in plasma after processing compared to the initial time point (T0h). Logically, if the failure was actually attributable to instability in blood, the percentage difference should be lower in the aged samples and decreasing with time. From the examples above, however, it seems there is another factor affecting the accuracy of the experiment, leading to the increased concentration of drug in plasma with respect to time.
Whole blood stability of Amlodipine and Celecoxib were performed at two different temperatures (ambient temperature and refrigerated) on the same day during method validation. The failure reported above only occurred for experiments performed under refrigerated conditions, but met acceptance criteria when conducted under ambient conditions. These data have proven that in the in vitro system, the equilibrium is impacted by temperature which is also different from in vivo situation, where the administered drug is already equilibrated in whole blood.
For the vancomycin example, when the aged time samples were compared with the initial time point (T0h), a large percentage difference was observed. Interestingly, the time point of 1 hour (T1h) compared with the subsequent time point (T2h), was only 2.5% difference. The interpretation of these results was that between (T0h) and (T1h), the drug was still equilibrating between RBCs and plasma, but between (T1h) and (T2h), the drug was stable
Alternative experimental approaches for whole blood stability assessment
As described above, prior to method validation, the method development team should test the whole blood stability with the analyte of interest and understand the partitioning and customize the experimental approach accordingly to better reflect the behavior of the drug in vivo. Alternative experimental approaches deigned at BioPharma Services Inc., to differentiate drug stability in whole blood versus drug equilibration between whole blood fractions (RBCs and Plasma) have included but not limited to the following:
1. Modified Matrix Approach
In this approach of whole blood stability assessment, the experiment was modified where the whole blood is spiked at LQC and HQC level then warmed to 37℃ for 30 minutes. The whole blood is then split into 6 aliquots and stored under refrigerated conditions. After one hour, another fresh blood was spiked at LQC and HQC level, then warmed to 37℃ for 30 minutes.
Subsequently, the aged and freshly spiked whole blood samples were extracted instead of plasma using a slightly modified extraction procedure. This was required to differentiate drug stability in whole blood versus drug equilibration between whole blood fractions (RBCs and Plasma).
2. Modified Equilibration Approach
In this alternative approach of whole blood stability assessment, an extra time point (T3h) was included in the experimental design and analyte concentrations compared with the sample that has been aged for 1 hour (T1h). This approach is used to allow extra time for the drug to fully equilibrate between red blood cells and plasma proteins. This alternative approach could reduce variability during method validation, as the only change was the additional time point added and this is conducted in plasma rather than a modified matrix approach described above.
Why Choose BioPharma Services?
Whole blood stability is an important component of bioanalytical method validation and is regulatory requirements to support clinical or pre-clinical sample handling, prior to sample processing in the bioanalytical lab. It is acknowledged that establishing whole blood stability can possess unique challenges, often not due to drug instability, but rather drug equilibration between whole blood constituents. Alternative experimental approach have been developed and utilized when required at BioPharma Services to support studies with a pharmacokinetic endpoint.
Written by: Wu Pak (Anson) Kwan, Hongzhi Liu and Dr. Nicola Hughes.
Find out why BioPharma might be the right partner for you! Learn more about BioPharma Services and the wide array of bioanalytical services we provide.
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials 1/2a and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.