Bioanalytical Method Development: Focus on Prodrugs
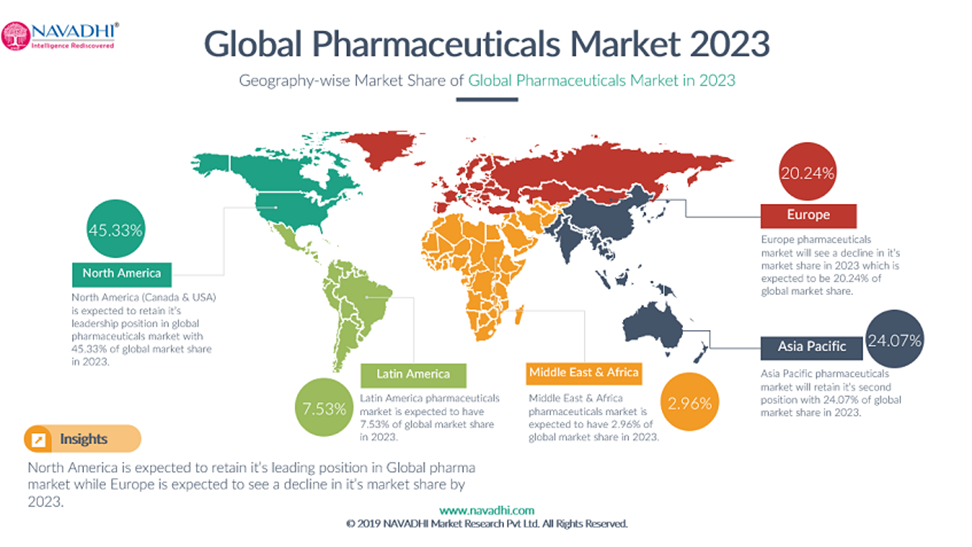
As described in the “Global Pharmaceuticals Industry Analysis and Trends 2023” report by NAVADHI Market Research, the Global pharma market is expected to be worth USD 1.57 trillion by 2023. North America is expected to retain its leading position in the global pharma market, with a market share of 45.33% in 2023. Asia Pacific pharma market is expected to retain its second position with a market share of 24.07% in 2023.
Of note, of the available active pharmaceutical ingredients in the world are, 10% are actually prodrugs. Over the past decade, the United States Food and Drug Administration (FDA) have approved around 30 prodrugs, around 12% from the approved new small-molecule entities.
-
What is a prodrug?
The International Union of Pure and Applied Chemistry (IUPAC) defines a prodrug as a chemical which requires a transformation prior to exerting its pharmacological effects. In other words, a prodrug is a “precursor” for the intended drug substance. Upon administration, the prodrug is converted to the desired active pharmaceutical molecule through an in vivo bioactivation processes (drug metabolism).
The concept of a prodrug was first defined in the late 1950s, although prodrugs have existed for more than a century. In fact, aspirin was the first prodrug that was marketed in 1899 and has been used since then for various therapeutic indications. Once aspirin enters the body, it is hydrolyzed into a substance called salicylic acid, which is the active metabolite.
How do prodrugs work, and why do we need them?
Prodrugs possess functional groups that can be biologically transformed by enzymes to be the same as the active medications. When a prodrug is taken orally, it enters the liver by a first pass mechanism. A common enzyme, cytochrome P450, which is mainly found in the liver, is predominantly responsible for “activating” the prodrug.
There are two general benefits of prodrugs, the first is that it improves a medication’s effectiveness by enhancing absorption (bioavailability) and thereby allowing the medication to reach its intended therapeutic site of action. The second benefit is that it reduces a medication’s toxicity or side effects.
Challenges on bioanalysis of prodrug in human fluids:
The most common prodrugs are ester-based, which were synthesized by derivatizing a phenol, hydroxyl, or carboxyl group on the intended active drug molecule. The cleavage process of an ester prodrug is catalyzed by various esterases in vivo to become its active “metabolite”. A challenge to bioanalysis is that the same hydrolysis reaction can also occur ex vivo, post-sample collection (which includes sample collection, processing, storage and thawing prior to extraction).
From a bioanalysis point of view, any ex vivo cleavage of prodrug molecules in biological matrix is undesirable. If this ex vivo cleavage is not controlled, it can lead to overestimation of the active drug molecule and by inference and underestimation of the residual prodrug concentration.
As a result, the safety and efficacy assessment of the prodrug and its active form, as a function of its concentration in biological matrix, will not be accurate and reliable. Over the past decade, bioanalytical scientists have made significant advances in understanding and minimizing this unwanted ex vivo enzyme-catalyzed cleavage of ester prodrugs in LC-MS bioanalytical testing.
Examples of Bioanalytical Methods developed and validated at BioPharma Services for Prodrugs
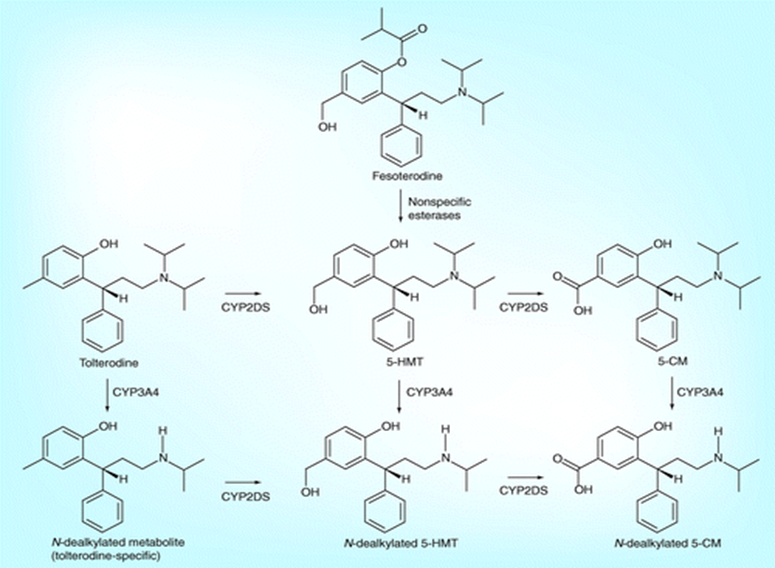
Prodrug Active drug form Acetylsalicylic acid Salicylic acid Dimethyl Fumarate Monomethyl Fumarate Fesoterodine 5-Hydroxymethyl Tolterodine Fingolimod Fingolimod Phosphate Lisdexamfetamine d-Amphetamine Psilocybin Psilocin Racecadotril Thiorphan Tenofovir alafenamide Tenofovir Case Study: Ultra-sensitive LC–MS/MS bioanalytical method development of fesoterodine (ester prodrug) and 5-hydroxymethyl tolterodine (5-HMT) in human plasma at BioPharma
Fesoterodine is a prodrug of a competitive muscarinic receptor antagonist. After oral administration, fesoterodine is rapidly and extensively hydrolyzed by nonspecific esterases to its active metabolite, 5-hydroxymethyl tolterodine, which is responsible for the antimuscarinic activity.
In support to a phase I study, a sensitive and selective LC-MS/MS method has been developed and validated for simultaneous determination of Fesoterodine and its active metabolite 5-hydroxymethyl tolterodine in human plasma. To avoid the ex vivo cleavage of fesoterodine during the sample collection, processing, storage and thawing prior to extraction, an enzyme inhibitor is required immediately after blood draw to stabilize fesoterodine.
In addition, control of temperature and pH are also required. Under these carefully optimized conditions, Fesoterodine and 5-HMT were stable for 2 hours in whole blood at 50C, for 18 hours in plasma and after 3 freeze-thaw cycles at -700C.
The stabilized plasma samples were extracted by Solid Liquid Extraction utilizing 200 µL of plasma sample, chromatography was performed on a Phenomenex Kinetex® EVO C18 analytical column (100 mm x 3.0 mm, 2.6 µm). The AB SCIEX QTRAP 5500 mass spectrometric instrument is operated in positive ion mode using a Turbo IonSpray source and MRM detection. The validated assay linear dynamic range of the method is from 2.00 to 400.00 pg/mL for Fesoterodine and 50.00 to 10000.00 pg/mL for 5-hydroxymethyl Tolterodine.
The method was fully validated, meeting the current requirements described in the ICH M10 guidelines for bioanalytical method validation, and has successfully applied to a phase I biostudy.
Some example chromatograms for the Ester prodrug – fesoterodine and 5-HMT
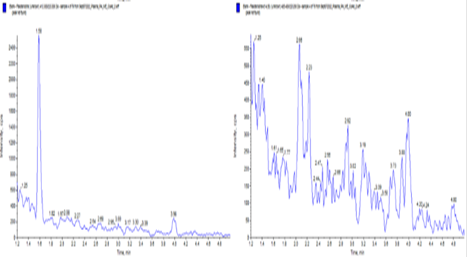
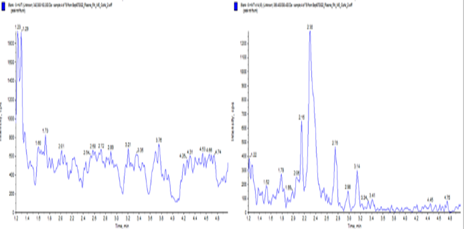
Fesoterodine extracted plasma Blank
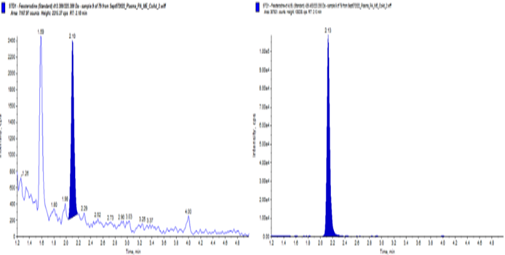
Fesoterodine extracted plasma LLOQ (2.00 pg/ml)
5-HMT extracted Plasma Blank
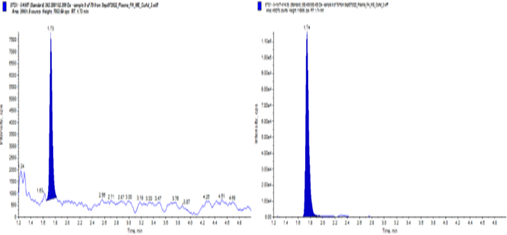
5-HMTextracted Plasma LLOQ (50 pg/ml)
Why Choose BioPharma Services?
BioPharma Services Inc. provides solutions for all your small molecule bioanalytical needs. Our state-of-the-art bioanalytical laboratory is fully equipped and staffed to perform individual assays and support your preclinical and clinical studies, including bioanalytical studies of prodrugs, with more than 250 validated assays available for a wide variety of therapeutic indications. Most importantly, we ensure the success of your projects by anticipating, understanding, and overcoming technical challenges ensuring high quality and compliance with regulatory guidance.
Written by: Wu Pak (Anson) Kwan, Hongzhi Liu and Dr. Nicola Hughes.
Find out why BioPharma might be the right partner for you! Learn more about BioPharma Services and the wide array of bioanalytical services we provide.
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials 1/2a and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.



