PBPK Modeling in Predicting Drug Behavior
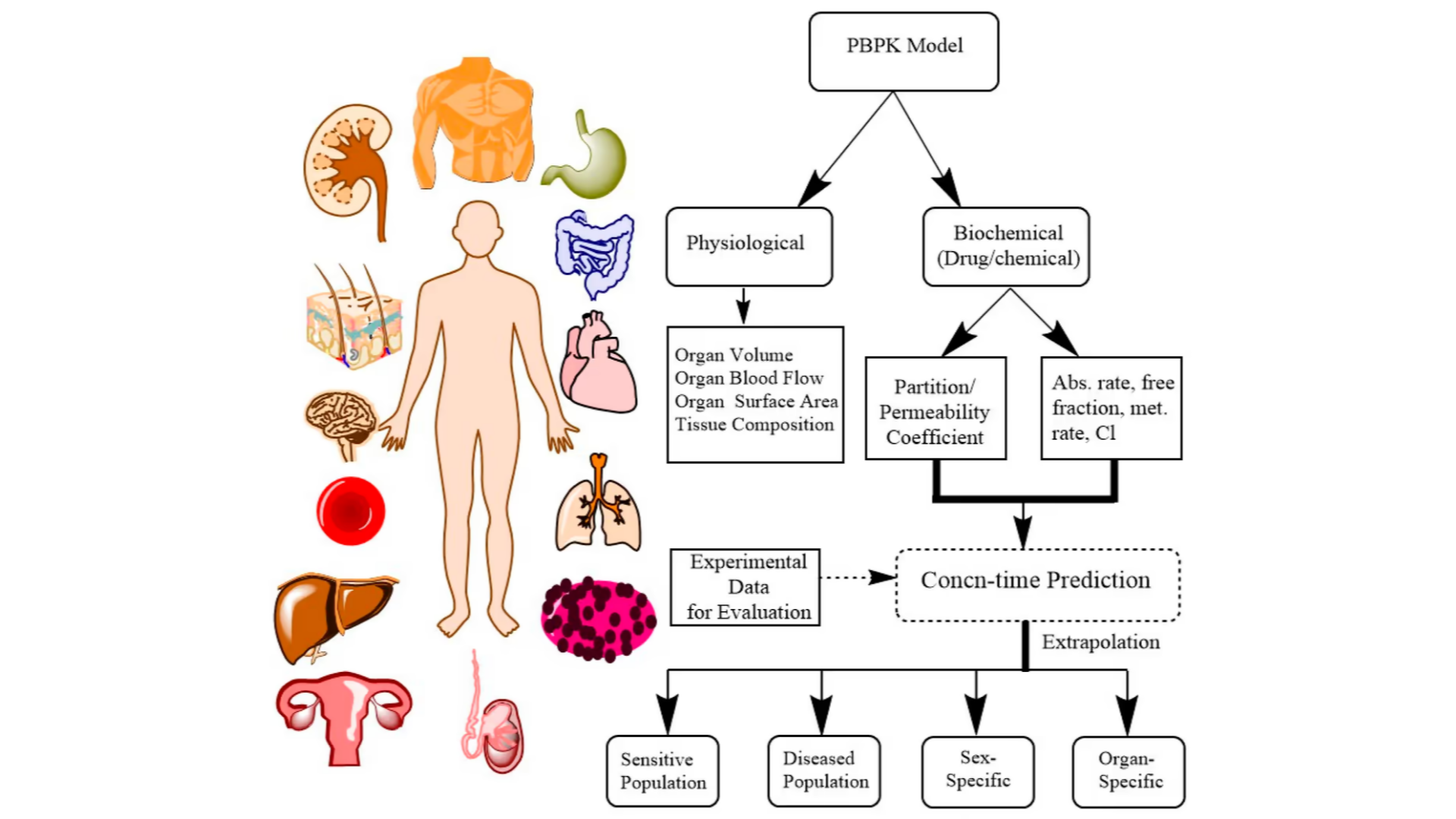
Physiologically-based pharmacokinetic (PBPK) is a mathematical modeling approach used in pharmacology and toxicology to predict the behavior of drugs in the body. PBPK models take into account the physiological and biochemical characteristics of the body, such as organ size, blood flow, and metabolic rates, to simulate how a chemical is absorbed, distributed, metabolized, and excreted over time.
General Procedures Used for PBPK Modeling
- Define the research question: The first step in any PBPK modeling project is to clearly define the research question or objective. This could be anything from predicting the pharmacokinetics of a new drug candidate in humans, to evaluating the impact of genetic variability on drug metabolism.
- Gathering Data: Once the research question is defined, the next step is to gather the data needed to build the model. This could include data on the drug’s physicochemical properties, pharmacokinetics in preclinical species, and clinical data on drug safety and efficacy.
- Development: The next step is to develop the model itself. This involves building a mathematical model that describes the absorption, distribution, metabolism, and excretion (ADME) of the drug in question. The model should be based on the physiological and biochemical processes that govern drug ADME, and should incorporate data on the drug’s physicochemical properties and pharmacokinetics.
- Validation: Once the model is developed, it must be validated to ensure that it accurately predicts drug pharmacokinetics and pharmacodynamics. This can be done by comparing the model’s predictions to clinical data from human trials or to preclinical data from animal studies.
- Use for Predictions: Once the model is validated, it can be used to make predictions about drug pharmacokinetics and pharmacodynamics in various populations. For example, the model might be used to predict the impact of drug-drug interactions, or to evaluate the effect of genetic polymorphisms on drug metabolism.
- Interpret and communicate the results: The final step is to interpret and communicate the results of the modeling project. This might involve writing a report or publication summarizing the modeling results, or presenting the results at a scientific conference or meeting.
The Usage of PBPK Modelling
The physiologically-based pharmacokinetics has several important uses in drug development and regulatory decision-making.
One of the main uses of PBPK is to predict drug exposure levels in different tissues and organs of the body. By taking into account factors such as drug properties, patient/subject characteristics, and physiological processes, PBPK models can provide a relatively accurate prediction of how drugs will behave in the human body, and further help researchers and clinicians optimize dosing regimens to achieve therapeutic levels in the target tissue, and evaluate the potential risk of toxicity associated with a drug being developed. This can be particularly important for drugs with narrow therapeutic windows or that are known to cause toxicity in certain organs.
PBPK modeling can also be used to predict drug-drug interactions. When multiple drugs are taken simultaneously, they can interact with each other and affect the pharmacokinetics and pharmacodynamics of each other. PBPK models can help predict these interactions and inform dosing regimens to minimize adverse effects or maximize therapeutic efficacy.
In addition, PBPK can be used to evaluate the safety and efficacy of new drug formulations or routes of administration. By simulating drug distribution and metabolism in the body, PBPK models can provide insight into the pharmacokinetic profiles of different formulations or delivery methods, which can help inform decisions about which formulation or route of administration to use in clinical trials.
PBPK models can also play a role in regulatory decision-making. For example, PBPK can be used to predict the exposure levels of a drug in different populations, such as pediatric or elderly patients, which can help inform decisions about dosing and safety. PBPK can also be used to evaluate the bioequivalence of generic drugs, which is important for ensuring that generic drugs are as safe and effective as their brand-name counterparts.
Since PBPK modeling allows researchers to simulate drug ADME in the body with the consideration of physiological parameters such as organ blood flow, tissue permeability, and drug-specific properties such as solubility, permeability, and protein binding. Such models can also be used to predict the pharmacokinetics (PK) of a drug in different populations (e.g., healthy subjects) and under different conditions (e.g., fasted, fed). By integrating PBPK models with pharmacodynamic (PD) models, it is possible to predict drug efficacy and safety to use for Virtual Bioequivalence (VBE).
Virtual bioequivalence (VBE) studies are becoming increasingly popular in the pharmaceutical industry. These studies use in-silico modeling to predict the bioavailability of a drug product, rather than conducting an actual bioequivalence study. PBPK modeling is a key component of VBE studies, as it provides a means to simulate drug absorption, distribution, metabolism, and excretion (ADME) in a virtual population. In this blog, we will discuss how PBPK modeling is used in VBE studies.
Regulatory Guidance on PBPK Modelling
Regulatory guidance on the use of physiologically-based pharmacokinetics (PBPK) varies by country/regions regulatory agencies. Here are some examples of current guidance from different regulatory agencies:
In the United States, the Food and Drug Administration (FDA) has issued several guidance documents that mention the use of PBPK. For example, the FDA’s guidance on “Waiver of in vivo Bioavailability and Bioequivalence Studies for Immediate-Release Solid Oral Dosage Forms Based on a Biopharmaceutics Classification System” encourages the use of PBPK modeling to predict the bioavailability of certain drugs.
The FDA’s guidance on “Physiologically Based Pharmacokinetic Analyses — Format and Content” provides recommendations for the use of PBPK modeling in regulatory submissions, including the types of data that should be included in the model and the sensitivity analysis that should be performed. There have been studies using PBPK models to waive the potential of many DDI studies approved in US.
In the EU, the European Medicines Agency (EMA) has also issued guidance on the use of PBPK. The EMA’s guidance on “Guideline on the use of pharmacokinetics and pharmacodynamics in the development of antimicrobial medicinal products” recommends the use of PBPK modeling to predict the pharmacokinetics of antibiotics in different patient populations. The EMA’s guideline on “Model-informed drug development: focus on paediatric pharmacology” encourages the use of PBPK modeling to predict drug exposure in pediatric patients.
In Japan, the Pharmaceuticals and Medical Devices Agency (PMDA) has issued guidance on the use of PBPK. The PMDA’s guidance on “Guidance on Submission of Drug Interaction Study Reports” recommends the use of PBPK modeling to predict drug-drug interactions.
Example of PBPK Modelling
Here are a few examples of how PBPK modeling can be used to support a waiver for a drug-drug interaction (DDI) study in a regulatory submission.
Let’s say that a drug company is developing a new drug that is metabolized by the cytochrome P450 (CYP) 3A4 enzyme, and they want to market the drug for use in combination with another drug that is known to inhibit CYP3A4. Normally, a DDI study would be required to evaluate the potential interaction between the two drugs and to determine whether the dose of the new drug needs to be adjusted when given with the CYP3A4 inhibitor.
However, if the drug company can show through PBPK modeling that the interaction between the two drugs is minimal, they may be able to request a waiver for the DDI study. To support this waiver, the drug company would develop a PBPK model that takes into account the pharmacokinetics of both drugs, the metabolism and inhibition of CYP3A4, and any other relevant factors.
The PBPK model would then be used to simulate the pharmacokinetics of the two drugs when given together, and to compare the results to the pharmacokinetics of the new drug alone. If the PBPK model predicts that the interaction between the two drugs is minimal and that the dose of the new drug does not need to be adjusted when given with the CYP3A4 inhibitor, the drug company may be able to request a waiver for the DDI study.
This type of approach has been successfully used in regulatory submissions for drugs such as midazolam and simvastatin. By using PBPK modeling to support a waiver for a DDI study, drug companies can save time and resources and potentially bring their drugs to market more quickly. However, it is important to note that the use of PBPK modeling to support a DDI study waiver must be justified by strong scientific evidence and must be approved by regulatory authorities. Osilodrostat, for example, when using PBPK approach predicting DDI effect was part of a New Drug Application (NDA) submission.
Software Used for PBPK Modelling
There are several software programs that are commonly used for PBPK modeling. Here are a few examples.
-
- Simcyp: Simcyp is a commercial software package that is widely used in the pharmaceutical industry for PBPK modeling. It includes a comprehensive library of physiological parameters and drug-specific data, and allows users to simulate drug absorption, distribution, metabolism, and excretion in virtual patient populations.
- GastroPlus: GastroPlus is a commercial software package developed by Simulations Plus. It includes a range of modules for PBPK modeling, drug-drug interaction prediction, and formulation optimization. It also includes a comprehensive database of physiological and drug-specific parameters.
- PK-Sim: PK-Sim is a freely available software tool developed by the Open Systems Pharmacology (OSP) project. It provides a platform for PBPK modeling and simulation, and includes a range of physiological models, drug-specific data, and built-in algorithms for drug metabolism and transport.
The Cost of Using PBPK Modeling
The cost of a PBPK modeling project can vary widely depending on the complexity of the model, the scope of the project, and the expertise of the modeling team. PBPK modeling projects can range from simple models with a few compartments to complex models with dozens of compartments that simulate multiple organs and physiological processes.
In general, PBPK modeling projects are labor-intensive and require specialized expertise, so they can be relatively expensive. The cost of a typical PBPK modeling project can range from a few thousand dollars to several hundred thousand dollars, or more, depending on the factors mentioned above.
The cost of a PBPK modeling project may also depend on whether the project is being conducted in-house or outsourced to a third-party modeling service provider. In-house modeling may require an initial investment in software, hardware, and personnel training, whereas outsourcing can provide access to specialized expertise without the need for these investments.
It’s worth noting that the cost of a PBPK modeling project can be offset by the potential cost savings for less clinical trials and risk reduction associated with improved drug development and safety assessments.
Harnessing PBPK Modeling: Key to Accelerated Drug Development
Overall, in the past decade, we have witnessed increased usage of PBPK modeling & simulation to assist the drug development. Such innovative approaches in mechanistic assessment of absorption, distribution, metabolism, and excretion (ADME), pharmacology, physiologically based modeling, and regulatory science have enabled a mindset from risk aversion to informative prescribing guidance for optimized development. The main usage of PBPK is to provide insight into the pharmacokinetics of drugs in the body and to inform drug development and clinical decision-making, and can apply to both NCE and generic drug development. PBPK modeling is a powerful tool that can help researchers and clinicians optimize drug dosing, predict drug-drug interactions, evaluate new drug formulations, and inform regulatory decision-making.
In an era where the demand for more precise and personalized medicine is ever-growing, PBPK modeling presents an innovative and scientifically rigorous approach to meet these challenges. Through its potential to improve drug development efficiency and reduce risk, PBPK modeling holds promise for enhancing the overall drug development process and patient care. As we move forward, we anticipate the continued advancement and broader acceptance of PBPK modeling in pharmaceutical research and regulatory applications, paving the way for improved therapeutics and patient outcomes.
Why Choose BioPharma Services?
At BioPharma Services, our expert pharmacokinetics team, specializing in PBPK modeling, can provide robust support to drug development sponsors. Leveraging our team’s proficiency in data management and analysis, we can handle complex PBPK models, process diverse sets of data, and ensure model validation against clinical or preclinical trials. BioPharma Services strictly adheres to regulatory guidelines from global health authorities, facilitating accurate prediction of drug-drug interactions and simulating drug behavior in various populations. Our team’s proficiency in PBPK modeling software tools, such as Simcyp, GastroPlus, and PK-Sim, further bolsters our services. By partnering with us, sponsors can achieve cost-efficiency, avoiding initial investments in software, hardware, and training, while gaining access to specialized expertise that aids in informed decision-making for their drug development projects.
Written By: Juan He, Vice President, Pharmacometrics.
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials 1/2a and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.



