FIX-DOSED COMBINATION: APPROACHING AN OLD QUESTION WITH A NEW CONCEPT
The development of generic fixed-dose combination (FDC) has seized increasing industry interest due to its advantages in disease management, patient compliance, and market benefits. For a generic FDC submission, the innovator has established the first bridge between FDC and co-administration of mono-components (mono-components), and the generic sponsor only demonstrates the second bioequivalence (BE) bridge between generic and innovator FDCs.
However, these two BE bridges increase the risk of bioavailability drifting from original mono-components to generic FDC and regulatory agencies can require evidence to prove the absence of “drifting” bioavailability.
Understanding Bioavailability; drifting from original mono-components to generic FDC
Determining this phenomenon by a direct Bioequivalence study between a generic FDC with the combination of medicinal products poses complicated issues in practice due to multiple comparison bridges, large numbers of FDC strengths, difficulty in securing the right reference listed drug (RLD) for each regulatory agency, discontinued RLDs, and ethical issues of exposing more healthy subjects to unnecessary medication.
To substitute direct comparison studies, Adjusted Indirect Comparison (AIC) is the most appropriate and feasible approach, which utilizes BE data from published articles to assess their equivalence. This abstract used published data to validate the AIC method and its role in assessing drifting bioavailability, cutting development costs, and shortening the time for generic FDC to market.
How AIC Aggregates data
The AIC uses aggregated data (i.e., T/R ratios and CIs) from BE studies to compare the interventions of interest in adjusting the results with a common treatment group, enabling a direct assessment of bioavailability drift between generic FDC and mono-components.
An AIC analysis was performed with two BE studies: the 1st BE bridge (the original BE study of innovator FDC vs. co-administration of mono-components, i.e., AB vs. A+B) and the 2nd bridge (the inherent BE study of generic FDC vs. the innovator FDCs, i.e., A’B’ vs AB).
1st BE bridge: AB = A+B <- inversed -> A+B = AB
2nd BE bridge: A’B’ = AB
AIC result: A’B’ = A+B
To demonstrate the validity of AIC in assessing BE drift, the results of the AIC analysis were subsequently validated with the published direct comparison study, which assessed generic FDC and mono-components.
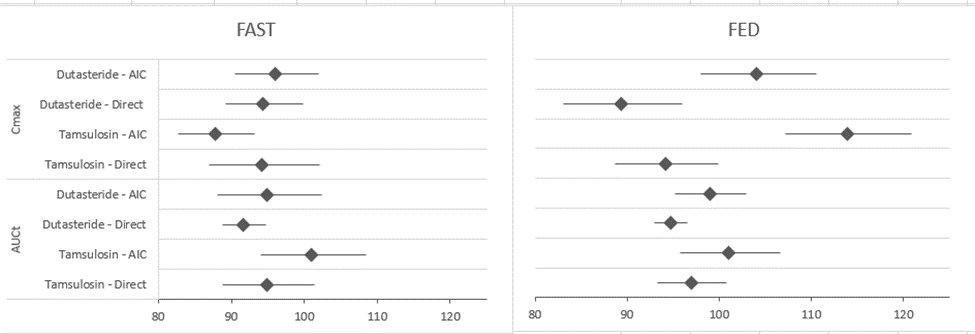
The Test/ Reference ratio and its corresponding 90% confidence interval of Cmax and AUCt for Dutasteride and Tamsulosin using AIC and direct comparison.
Therefore, not only is AIC known as an appropriate methodological approach that utilizes available data from the literature to accelerate generic FDC development and support interchangeability between generics effectively, but it also reinforces the safety and efficacy of generic FDCs by assessing the potential bioavailability drift without additional development cost.
How BioPharma can help your next FDC submission?
BioPharma Services has diverse experience with novel and generic FDC products submitted to different regulatory such as FDA, EMA, TPD, GCC, TGA, ANVISA, NMPA, and more. Our Scientific group collaborating with other core teams in the Clinic and Bio-Laboratory can support you from the beginning of product development plan and study design, study conducting, bioanalytical testing, data analysis, until report writing.
Written by Sunny Le – Credited to Thuy Van Nguyen and Jacinda Kwok
Find out why BioPharma might be the right partner for you! Learn more about BioPharma Services and the wide array of bioanalytical services we provide.
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials 1/2a and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.