How to Conduct Human Clinical Trials

Learn about all aspects of Human Clinical Trials
Click to jump to a section:
The process of conducting human clinical trials is long, systematic, and rigorous. Using a clinical trial services company like BioPharma Services ensures the quality of all aspects of your project. These aspects include but are not limited to your trial’s scientific affairs, medical writing, pharmacokinetics, biometrics/CDISC, clinical trial data management, and recruitment.
Clinical trials on human subjects evaluate new drugs, devices, treatments, or diagnostics as well as the safety, effectiveness, benefits, costs, adverse effects, and outcomes of approved medicines, devices, and treatments. They are conducted as a part of the U.S. Food and Drug Administration (FDA) approval process for new drugs or devices, simultaneously with an Investigational New Drug (IND) Application or as IND exceptions for drug studies. In addition, it is essential to understand the distinctions and intersectionality among the markets that the FDA and other regulatory agencies, such as the European Medicines Agency (EMA), Health Canada, and the Pharmaceuticals and Medical Devices Agency (PMDA) of Japan, cover.
In all cases, as is with us at BioPharma, studies should include intended human enrollment and controlled testing of drugs, devices, treatments, or diagnostic tools following approved protocols.
Our human clinical trial research follows strict scientific standards and guidelines aimed at:
- Participant protection and safety
- Realization of accurate and productive results
The Importance of Human Clinical Trials
Human clinical trials provide critical evidence to improve patient care and overall healthcare. Clinical studies partnered with clinical trial services companies help physicians to evaluate:
- The new approach’s effectiveness and safety for patients
- The most effective treatments or strategies for specific diseases and groups of people
Testing should be conducted following a procedure or protocol that describes the objectives, design, and methodology of a clinical study as well as the relevant scientific background. Vital information to add into a protocol includes:
- The participant number and eligibility
- Test time, duration, and regularity
- Data collection types
- Human clinical trial duration
- Therapy plan details
This information can be expertly compiled with a clinical trial services company, enabling you to focus on your research.
The Phases of Human Clinical Trials
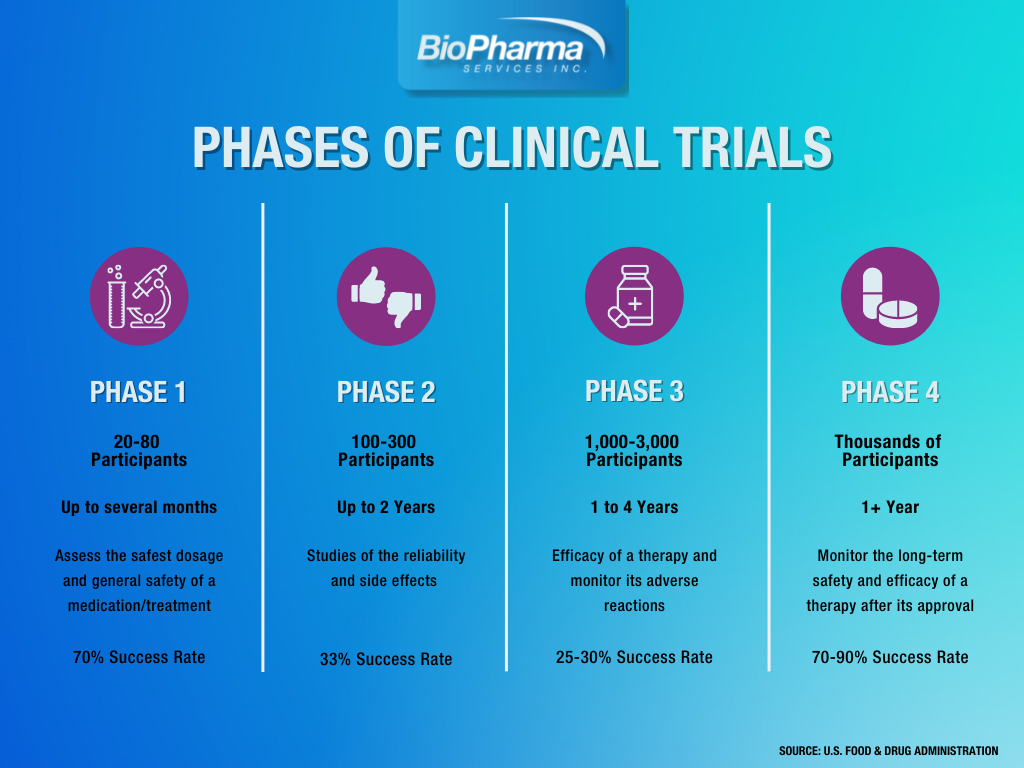
Human clinical trials provide crucial information on how treatments cost-effectively affect the patients’ quality of life and on the diagnostic value of tests. There are four phases of a complete clinical trial: Phase 1, 2, 3, and 4. Phase 1 clinical trial studies ascertain the safest dosage and general safety of a therapy, whereas Phase 2 trials establish its reliability and side effects. Phase 3 studies determine the efficacy of a therapy and monitor its adverse reactions. Finally, Phase 4 trials monitor the long-term safety and efficacy of a therapy after its approval.
The Characteristics of Early-stage Clinical Trials
Phase 1 Clinical Trials
- Study participants: 20–100 healthy individuals or, in some cases, people with the studied disease
- Duration: Multiple months
- Objective: Ascertaining the safest dosage and general safety of the therapy
- ~70% of therapies advance to the following phases
First-in-Human Studies
- A Phase I study type
- Represents the first administration of a preclinically tested study drug in humans
- Determining the initial dose and dose acceleration schedule is key
Phase 2 Trials
- Study participants: Up to hundreds of people with the studied disease
- Duration: Multiple months to 2 years
- Objective: Establishing the reliability and side effects of the therapy
- ~33% of drugs advance
As a full-service clinical trial services company that specializes in Phase 1 and Phase 2a human clinical trials, the BioPharma team is with you throughout the entire process, from beginning to end.
Frequently Asked Questions (FAQ’s) About Human Clinical Trials

Where does it all begin?
Research often starts in laboratories, where new concepts are developed. Animal studies follow and allow scientists to discover the impact of these approaches on living organisms. Next, the therapy is tested in a small group of humans in Phase 1 clinical trials before progressing through all trial phases.
What level of quality control is necessary?
At BioPharma, we understand that writing study protocols can be arduous. Many clients come to us with written protocols that require modifications. After reviewing the client’s protocol, we swiftly communicate the necessary changes. The FDA has strict rules regarding protocol modifications after a protocol approval, and logistical changes can be challenging; thus, having a clinical trial services company by your side is the best decision you can make for your project.
In human clinical trials hosted by clinical trial services companies, testing can be done to:
- Analyze therapies for diseases, syndromes, and conditions related to the access to drugs, medical devices, or surgical or therapeutic options
- Analyze methods to stave off illnesses through using changes in patient lifestyle, vaccines, and medications
- Analyze or improve diagnostic tools
- Identify risk factors for diseases or conditions that need recognition
- Become familiar with supportive therapies to improve the quality of life of people with chronic conditions
The results of human clinical trials determine whether the new therapy:
- Improves a patient’s quality of life
- Causes an unexpected damage
- Has a neutral or negative effect on the patient
Who is on the research team?
Each clinical trial is led by a principal investigator, usually a physician. Besides the primary investigator, the research team may include other medical professionals, data administrators, nurses, a social worker, and a clinical research organization (CRO) coordinator.
Where are human clinical trials carried out?
Clinical trials can be carried out at CRO or clinical trial service company centers, hospitals, universities, medical centers, clinics, community clinics, or federal research organization facilities.
What is the duration of clinical trials?
The duration of clinical trials depends on their phase and scope. Most importantly, the clinical trial design determines the study’s duration, sample size, and organization.
Some processes may take days, whereas others may take years. As is good clinical practice (GCP), volunteers are informed before enrolling in the study regarding the length of time they will be required to dedicate to it.
Conducting a Human Clinical Trial With BioPharma
One of the features that sets us apart is our extensive expertise in early-Phase 1 clinical trials and abuse potential studies. Moreover, we are willing to take on challenging studies with various routes of administration and unique needs. When conducting your research with us, we are dedicated to recognizing and avoiding bias. Our clinical researchers can prevent bias by ensuring volunteer safety and using comparison groups, randomization, and masking.
Volunteer Safety
Recently, instances of unethical behavior of researchers have threatened public confidence, and the federal government is calling to strengthen clinical research rules and guidelines to protect participants from undue risks. Although researchers strive to manage the risks for clinical trial participants, there are inherent uncertainties in human clinical trials of new drugs.
Therefore, we at BioPharma believe it is critical to ensure volunteers have a well-rounded understanding of the steps they will take and potential risks before committing to participation in a trial.
Early-Phase Clinical Trials
Our scientific team has extensive expertise with early-stage Phase 1 clinical trials, including first-in-human (FIH) studies. We can help researchers identify a safe initial dose and an optimal schedule for dose acceleration in FIH trials. Our approach is tailored to the individual needs of our clients and is based on their previous experience and set of preclinical data. In addition to determining the study design for early-stage clinical trials, we can provide expert feedback and recommendations on all safety aspects of the study.
Human Clinical Trials on Special Populations
We have also conducted numerous studies on special study populations, including human abuse potential studies. A partnership with Cambridge Cognition has enabled us to streamline pharmacodynamic assessments using electronic capture and computerized evaluations. Our volunteer database encompasses more than 18,000 active participants, including healthy males and females, recreational drug users, and age- and gender-restricted populations, enabling efficient participant recruitment for our clients’ studies.
Phase 1 Centers
Our state-of-the-art Phase 1 centers are located in Toronto, Canada and St. Louis, Missouri. Their infrastructure enables us to comprehensively support our clients’ early-phase drug developmental needs. In addition, our in-house bioanalytical laboratory can conduct diverse bioequivalence (BE) and bioavailability (BA) studies.
Randomization, Masking, and Blinding
In statistical analysis, confounding factors can make determining the proper relationship between two properties difficult. This is yet another area in which we can provide guidance as your clinical trial services company. We control for bias in our trials through randomization with our proprietary computer program systems, especially in comparative group human clinical trials. Due to the participants’ random rather than voluntary assignment to comparison groups, differences observed during the study are due to distinct strategies rather than individual differences. By not revealing to participants or researchers which groups they belong to, masking or blinding helps avoid bias.
BioPharma Services — Your Clinical Trial Services Company
Human clinical trials are an integral part of discovering and developing new products and are required by the FDA and other regulatory agencies before products are brought to the market. “New” may mean “better,” but it is unclear whether potential treatments will benefit patients until clinical trials of the therapy are complete. Human clinical trials test possible treatments on human volunteers to determine whether their widespread use in the general population should be approved. Treatments can be drugs, medical devices or vaccines, blood products, or biopharmaceutical techniques.
While you embark on your journey to find the next best therapy, partnering with BioPharma Services as your clinical trial services company at every step of the way will help you streamline all aspects of your human clinical trials — from scientific affairs and medical writing to pharmacokinetics, biometrics/CDISC, clinical trial data management, and recruitment.
Find out why BioPharma might be the right partner for you! Learn more about BioPharma Services and the wide array of services we provide.
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials I/IIa and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.