SPOTLIGHT ON: Ryan Best – Director of Clinical Research

My First Job
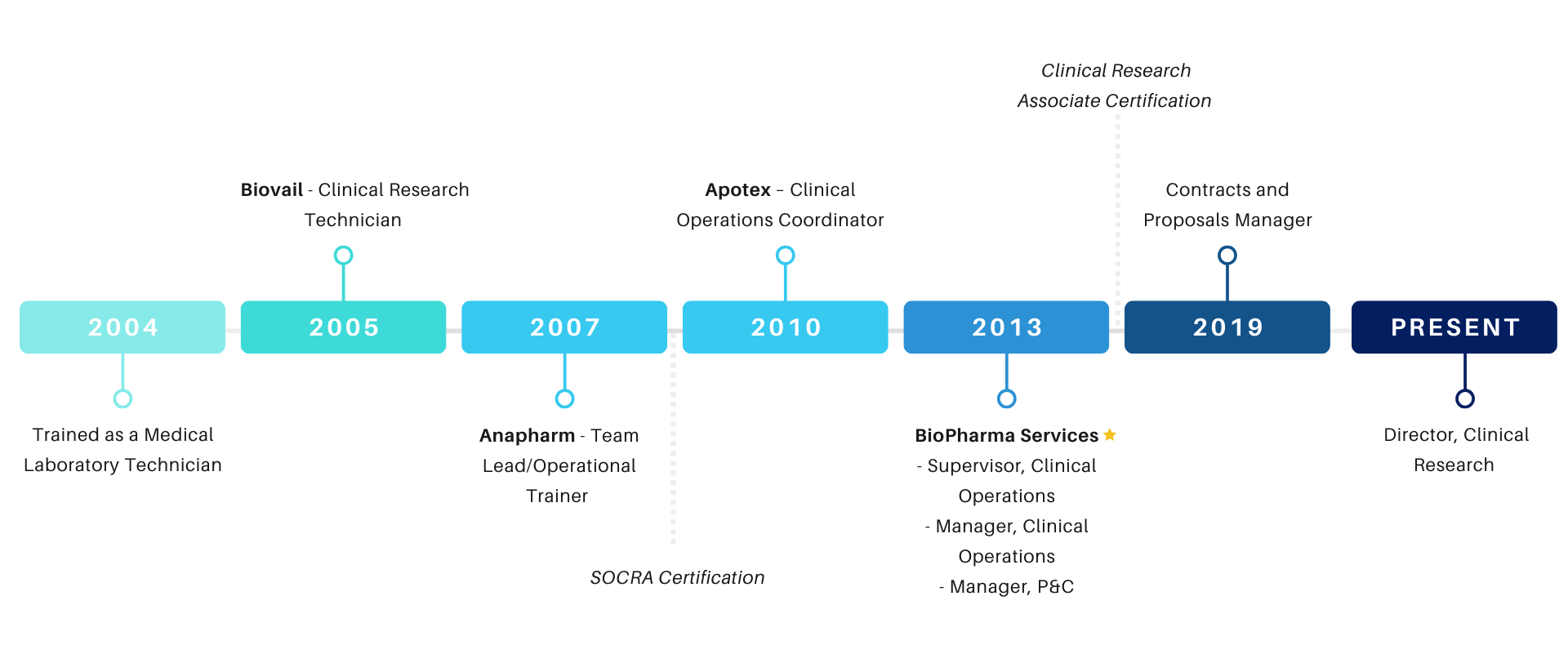
I completed my Medical Laboratory Technician course in 2004, and like many others in my graduating class, I aspired to begin my career in a hospital setting. My work in the Clinical Research field happened by chance. As it turned out, while driving to one of my first Hospital interviews, an advertisement played on the car radio for a company looking to compensate healthy males and females for participating in a clinical research trial.
I thought, why not explore this?
That would lead to my introduction to Biovail Contract Research Organization. After a bit of research, I scheduled a screening appointment to participate as a subject for a trial, and during my screening, I asked to speak with management and offered my resume. Within two weeks of my screening appointment, I was hired as a Clinical Research Technician. During the first three years of my clinical research career, I gained a firsthand understanding of the subject and technical staff experience. I do not doubt that this has played a significant role in my management career.
Joining a Larger Organization
After three years in my CRT role, I was eager and confident that I could take on a more challenging position, so in 2007 I joined a company new to Toronto, Anapharm Inc. as an Operational Trainer. When I joined Anapharm, they were in the early stages of preparing for their first clinical trials. My task as one of the Operational Trainers was to onboard, train, and evaluate the newly hired Operations team consisting of Physicians, Nurses, and Technicians. This role offered me a unique and integral perspective that I knew would help me grow in my Clinical Research career.
I joined Apotex Inc. as a Clinical Operations Coordinator in 2010 and moved from a training role to a coordinator role. I viewed this as an essential step in my career, since this position would allow me to play an integral role in the success of a trial. Not only that, but I spent three years with Apotex developing my coordinator skills and managerial abilities. Later, I obtained my certification with SoCRA as a Certified Clinical Research Professional during this period.
I was always motivated to improve myself, and I had the same motivation to improve the quality of my team and procedures and to be able to make an impact on a company. Since Apotex was such a large, well-established organization, it made it difficult for individuals to have an influence and play a role in process improvement. I knew I wanted more and could offer more. I needed to be in a place that welcomed change and had a growth mindset, so when BioPharma Services Inc. approached me to Supervise the Clinical Operations team, I eagerly accepted the opportunity.
The Start of my Clinical Research Career at BioPharma Services
Not only that, but I was inspired by the fact that BioPharma Services was a smaller, relatively young company. At this point in my career, I knew I was ready to join an organization where I could make positive changes by drawing on my observations from my nine years of experience in clinical research and the varied roles I had assumed. BioPharma Services offered me a position as Supervisor of Clinical Operations. I took immense pride in this opportunity, and I aimed to meet their expectations and more.
This position was followed soon after by a promotion to Senior Manager of Clinical Operations, allowing me to lead and assist in the development and growth of the Recruiting and Screening teams, while collaborating with the other functional leads to push for continuous improvement of the existing processes and procedures. In 2015, intending to continue my education, I completed my Clinical Research Associate Certification. My continued growth in my leadership role coincided with BioPharma’s continuous growth as a company as we took on more complex BA/BE, Phase 1 study designs, and our first Human Abuse Liability projects.
In 2019, I was approached by BioPharma Services management with an opportunity to try something completely new in a role that, at the time, I considered to be well outside my comfort zone.
I thought that change can be a positive experience.
As I inquired in more depth and analyzed this new role, I realized that this opportunity would offer me an entirely different, yet valuable, perspective on my clinical research work. Joining the Business Development Team as a Proposals and Contracts Manager required me to take an in-depth look at the financial implications of the clinical conduct decisions I had made over the past 15 years. I would gain a deeper understanding of how those operational choices could impact the profitability of a trial.
This role would give me invaluable insight. My heart was in the clinic, working alongside and supporting the frontline team, but I knew that the knowledge and awareness I would gain from this position would inevitably help them and the company. I trusted that one day I would return to an Operations role and utilize my experience and knowledge from this new position.
The mentoring, support, and guidance I obtained from the Senior and Executive management teams over the past nine years have undoubtedly played a crucial role in my success with BioPharma Services.
The Road Ahead
Earlier this year, I began the next phase of my BioPharma journey as the Director of Clinical Research. I bring to this position what I consider to be a well-rounded range of experience, and I am excited to see where my continued desire to learn and build strong relationships will take me in the years to come. BioPharma Services has been a great place to work ever since I started. They are always seeking bright minded individuals to join our team and if you want to join the exciting world of clinical research, BioPharma Services can be the best place to start. If you’re interested and want to join my team, or the many like it, visit our Careers page to learn more about our openings. Don’t see an opening that quite suits you, fill out our Contact Form and let us know your interest.

Find out why BioPharma might be the right partner for you! Learn more about BioPharma Services and the wide array of bioanalytical services we provide.
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials 1/2a and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.



