

The Role of a Clinic Pharmacy within a CRO
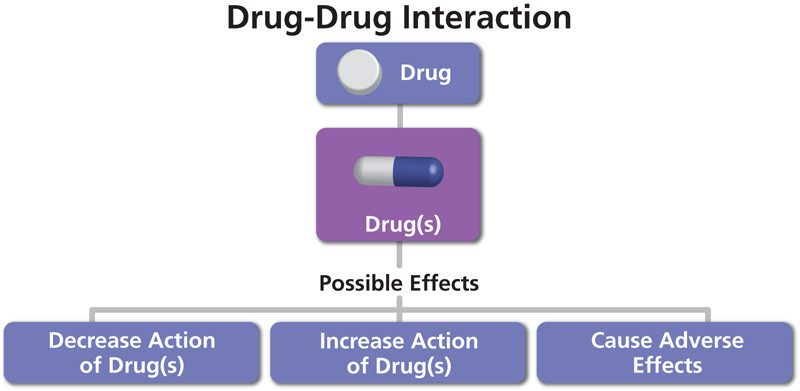
The Role of a Clinic Pharmacy within a CRO The clinic Pharmacy within a Clinical Research Organization (CRO), plays a fundamental role in the successful execution of a study. In collaboration with the trial sponsor, and the entire BioPharma Services team, the clinic...
Which Requirements Must Be Met to Conduct First-in-human Clinical Trials?
Which Requirements Must Be Met to Conduct First-in-human Clinical Trials? First in Human Clinical trials (FIH trials) play a critical role in bringing investigational new drugs or interventions to the clinical practice. To ensure the safety of study participants, FIH...
Canada: an Alternate Regulatory Route for First in Human Trials
Canada: an Alternate Regulatory Route for First in Human Trials The Pre-IND meeting is a critical meeting for any sponsor wanting to pursue a new drug approval (NDA) in the United States. With the ultimate aim to get the first approval of a drug in the large US...
Corrective Action Preventive Action (CAPA) Best Practices
Corrective Action Preventive Action (CAPA) Best Practices What is a CAPA (Corrective Action/Preventive Action) and why? A CAPA is a comprehensive investigation generated to address a systemic quality incident and is mandatory in resolving/preventing the issue from...

Recent Comments