Oncology Drugs in Clinical Trials with Healthy Populations Old Challenges or New Opportunities
PRESENTED TO: DIA 2023 by BioPharma Services
PRESENTED BY: D. YU, S. LE, J. HE & J. OLDENHOF
PURPOSE
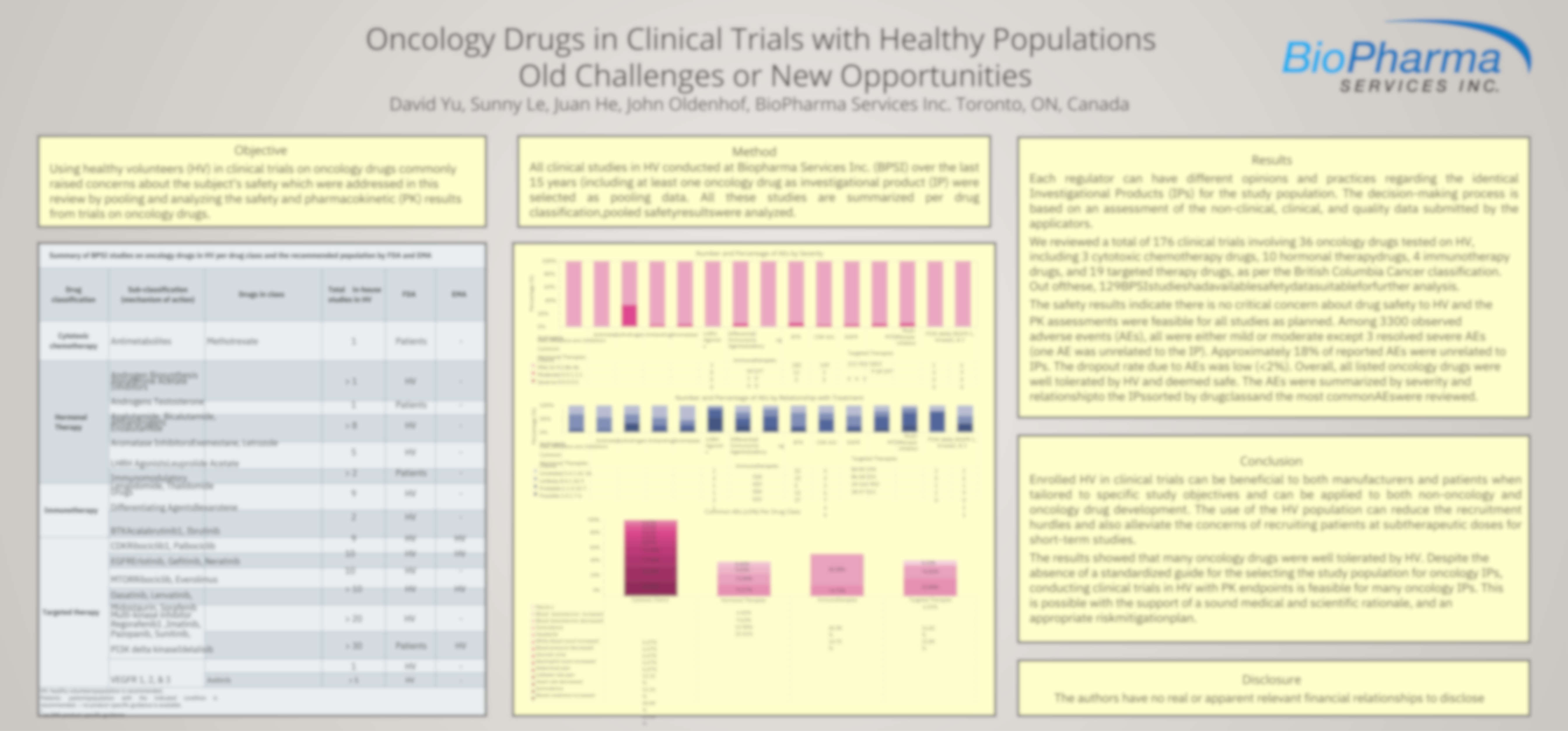
Using healthy volunteers (HV) in clinical trials on oncology drugs commonly raised concerns about the subject’s safety, which were addressed in this review by pooling and analyzing the safety and pharmacokinetic (PK) results from trials on oncology drugs.
To access the entire publication and view the full resolution PDF, please complete the form.