Comforts and Concerns in PK-based Bioequivalence Studies of Inhalation Products
PRESENTED TO: AAPS PharmaSci 360 Annual Meeting 2018
PRESENTED BY: N. Rayad, J. He.
PURPOSE
▪ Generic orally-inhaled products (OIPs) are warranted as safe, effective and affordable medications.
▪ Regulatory agencies – far from harmonized – require in vitro, in vivo PK, and/or PD studies to demonstrate bioequivalence (BE)/therapeutic equivalence (TE) to innovator products:
o FDA → weight-of-evidence approach (PK is one component).
o EMA → a stepwise approach (PK with charcoal blockade).
o Health Canada → aggregated evidences (similar to FDA’s).

OBJECTIVE(S)
1. Highlight the prominent PK properties of some OIPs.
2. Investigate the variability of OIPs in PK/BE clinical trials.
3. Summarize BPSI experience, designs and challenges in PK/BE.
4. Identify the safety profiles (in terms of AEs).
5. Highlight regulatory guidelines for these complex generics.
RESULT(S)
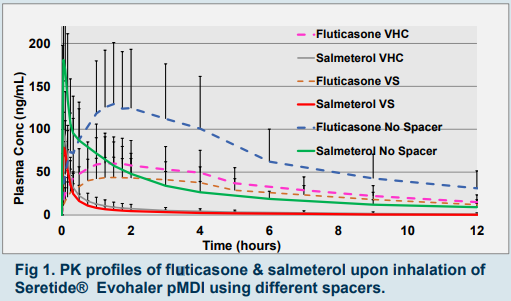
- Spacer for pMDIs
▪ VHC (AeroChamber plus valved holding chamber) & VS (Volumatic spacer); both:
→ reduced total systemic exposure by 38% and 68%, respectively compared to no spacer use (fig 1).
→ showed high inter-subject CV%, yet ISCV% was slightly lower for VS compared to VHC.
▪ VHC was superior to VS in terms of:
▪ Absorption (46% higher exposure; fig 1).
▪ Passing BE criteria. - Bioequivalence Study Design, PK profiles & BE comparisons
▪ Design: 2-way crossover; replicate design (for HVD); and two stage design (for attaining sufficient statistical power).
▪ T and R comparison → based on rate (Cmax and Tmax) & extent (AUCt) of absorption.
▪ Cmax → as early as 6 min with salmeterol, formoterol, budesonide and tiotropium; the Tmax was around 1h for fluticasone.
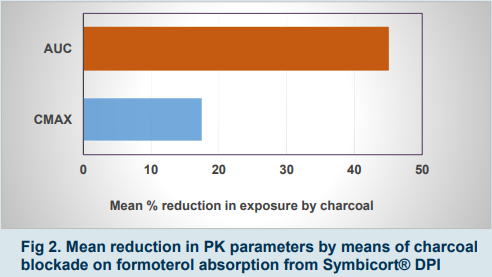
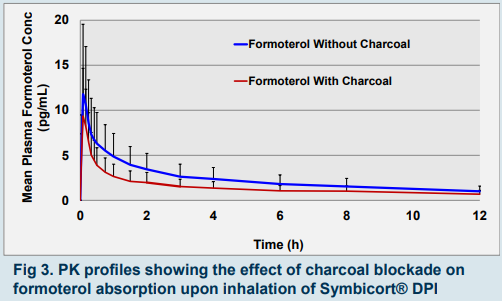
▪ Partial AUC0-30 → surrogate for efficacy if very quick lung absorption precludes significant gut absorption (e.g. salmeterol). - Charcoal Blockade resulted in:
▪ Formoterol: reduced enteral absorption of a proportion of inhaled drug (fig 2 and 3).
▪ Budesonide: almost same exposure as without charcoal.
Table (1): PK parameters (mean ±S.D.) of different inhalation drugs
| Inhalation Drugs | AUC0-30 (pg.h/mL) | AUinf (pg.h/mL) | Cmax (pg/ml) | Tmax (H) | Lambda (1/H) | T1/2 (H) |
| Budesonide | 230±160 | 1290±747 | 665±479 | 0.2±0 | 0.192±0 | 3.8±1 |
| Formoterol | 4±2 | 39±24 | 12±8 | 0.1±0 | 0.08±0 | 10±3 |
| Fluticasone | NA | 1400±565 | 193±68 | 0.9±0 | 0.07±0 | 13±2 |
| Salmeterol | 64±20 | 406±116 | 247±142 | 0.06±0 | 0.05±0 | 14±4 |
| Tiotropium | NA | 85±69 | 9±5 | 9±0 | 0.03±0 | 40±35 |
*AUCt is AUC 0-72
METHOD(S)
- Drugs studied were fluticasone, salmeterol, budesonide, formoterol, tiotropium bromide and combinations thereof.
- PK-BE studies were conducted on total of 580 NHVs; design/scope shown below:
Scope of PK/BE studies at BPSI
-
- Mono & combo inhalation products
- Metered-dose & dry-powder inhalers (pMDIs or DPIs)
- Charcoal blockade (with & without charcoal)
- Different types of spacers (VHC and VS)
- Two-way crossover, adaptive (2-stage) or fully replicate design
RECOMMENDATIONS
(Comforts & concerns – BPSI experience):
- Subjects and staff training and standardized inhalation technique were optimized for lung deposition of drug and reduced variability; such as the following:
→ MDI, a steady and gentle inhalation in coordination with actuation.
→ DPI, rapid, forceful and deep inhalation. - High variability: batch-to-batch variability; hooked on a formulation and active agent; the sample size and study design (adaptive, replicate and finally a 2-way crossover)



