Single and Multiple-Dose Bioequivalence Studies for Oxycodone Extended Release
PRESENTED TO: BioPharma Services Inc., Toronto, Ontario, Canada
PRESENTED BY: J. He, N. Gharavi, and F. Trabelsi
PURPOSE
The oxycodone extended release (ER) tablet (OxyContin® by Purdue Pharma L.P) provides long therapeutic duration for pain relief. The requirements of bioequivalence (BE) trials for generic drug submission for FDA and TPD require single-dose studies only, while EMA requires both, a multiple-dose study with the highest strength to demonstrate BE at steady-state, as well as a single-dose study.
OBJECTIVE(S)
Biopharma Services Inc. (BPSI) has conducted over 20 trials with doses ranging from 5 mg to 80 mg of oxycodone ER tablet using normal healthy volunteers (NHV). Four out of 20 studies were conducted as single-dose and multiple-dose trials using both the lowest (5 mg) and highest (80 mg) strengths. The objective of this presentation is to summarize pharmacokinetics (PK) and safety observations from in-house single-dose and multiple-dose trials.

METHOD(S)
• 4 pivotal studies
• Randomized, two-sequence, two-treatment, two-period, crossover trials in NHV
• 5 mg or 80 mg tablet strength (5 mg single-dose was fed study and no food effect was reported on PK of oxycodone
• Single-dose and Multiple-dose
• Safety was monitored throughout the course of the trial by evaluating reported adverse events (AE), clinical laboratory test results, vital signs, and ECG findings
• Blood samples for PK assessment were collected after oxycodone dosing
• Single-dose sampling schedule up to 36 hours
• Multiple dose sampling schedule (dosing interval 12 hours):
• Prior to the first dose of oxycodone administration;
• Pre-dose on Days prior to PK sampling day
• On PK sampling day up to 12 hours
• PK parameters such as maximum plasma concentration (Cmax) and area under concentration-time curve (AUC) were determined.
Table 1. Summary of studies conducted for single and multiple-dose administration
| Study | Days of Dosing | Dose Titration | Dose Reduction | Washout | Comment |
| 5 mg SD | 1 | No | No | Yes | |
| 5 mg SD | 5 | No | No | Yes | |
| 80 mg SD | 1 | No | No | Yes | Naltrexone given |
| 80 mg SD | 13 | Yes | Yes | No | Naltrexone given |
CONCLUSION(S)
PK was well characterized with relatively low variability of oxycodone under both single-dose and multipledose conditions
• No accumulation of oxycodone after multiple-dose administration
• More AEs observed for 80 mg than that from 5 mg (either single-dose or multiple-dose conditions)
• More AEs observed when multiple doses was given comparing to AE seen from a single-dose studies
• With proper safety protection, multipledose of 80 mg of oxycodone clinical trial was successfully conducted in NHV
• Our experience showed 80 mg multiple-dose trial was safe in NHV and scientifically sound to be discriminative to determine BE
RESULT(S)
All successful BE studies with 90% confidence intervals of geometric mean ratios of Cmax and AUC within 80.00% to 125.00%.
• The AEs observed in all single- and multiple-dose studies were either mild or moderate in nature.
• Rate of AE per subject was much higher in multiple-dose study (2.7%) than that from single-dose study (1%) for 80 mg strength
• The percentage of moderate AEs was also higher in multiple-dose trial than it observed from single-dose trial of both 80 mg and 5 mg strengths
Table 2. Summary of AEs Observed from both Single and Multiple-Dose Administration of 5 and 80 mg Oxycodone
| Study | Total AE | % AE/Subject | Mild AE | Moderate AE |
| 80 mg SD (n= 52) | 54 | 1 | 52 | 2 |
| 80 mg SD (n= 36) | 94 | 2.7 | 86 | 8 |
| 5 mg SD (n= 36) | 22 | 0.6 | 22 | 0 |
| 5 mg SD (n= 36) | 39 | 1.1 | 36 | 3 |
Similar Median Tmax: 2 to 4 hrs with the range of 0.5 to 6 hrs when given as both single-dose and multiple-dose administration with both 5 mg and/or 80 mg strength
• Mean T1/2 of oxycodone is relatively short as 5 hrs after single-dose administration
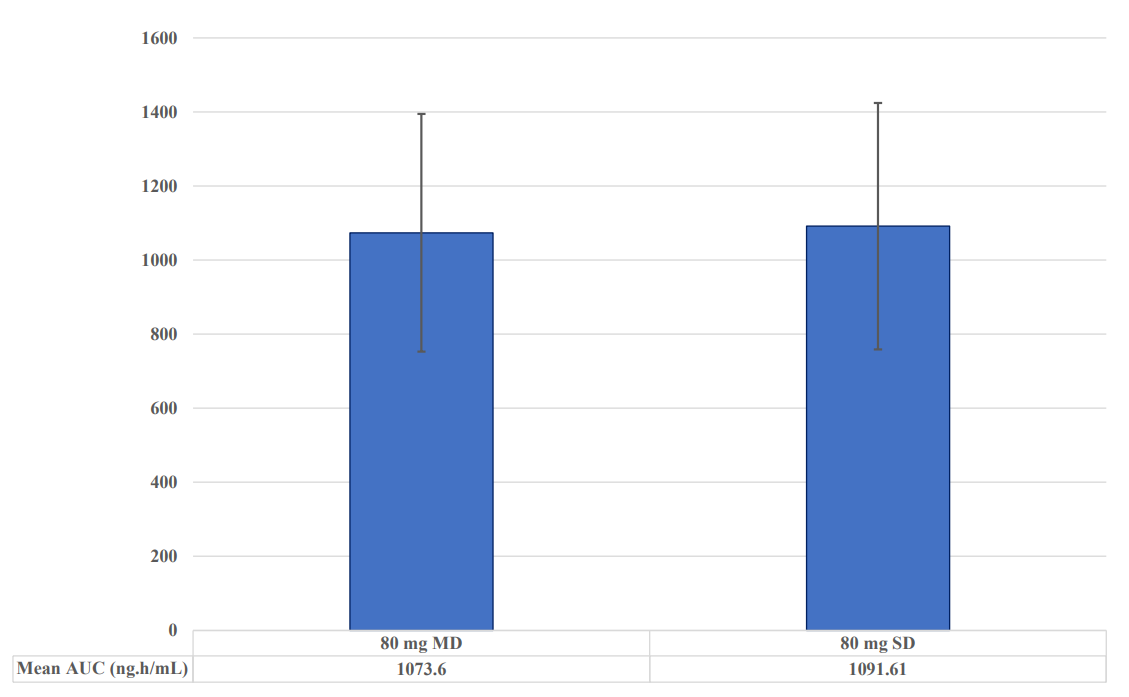
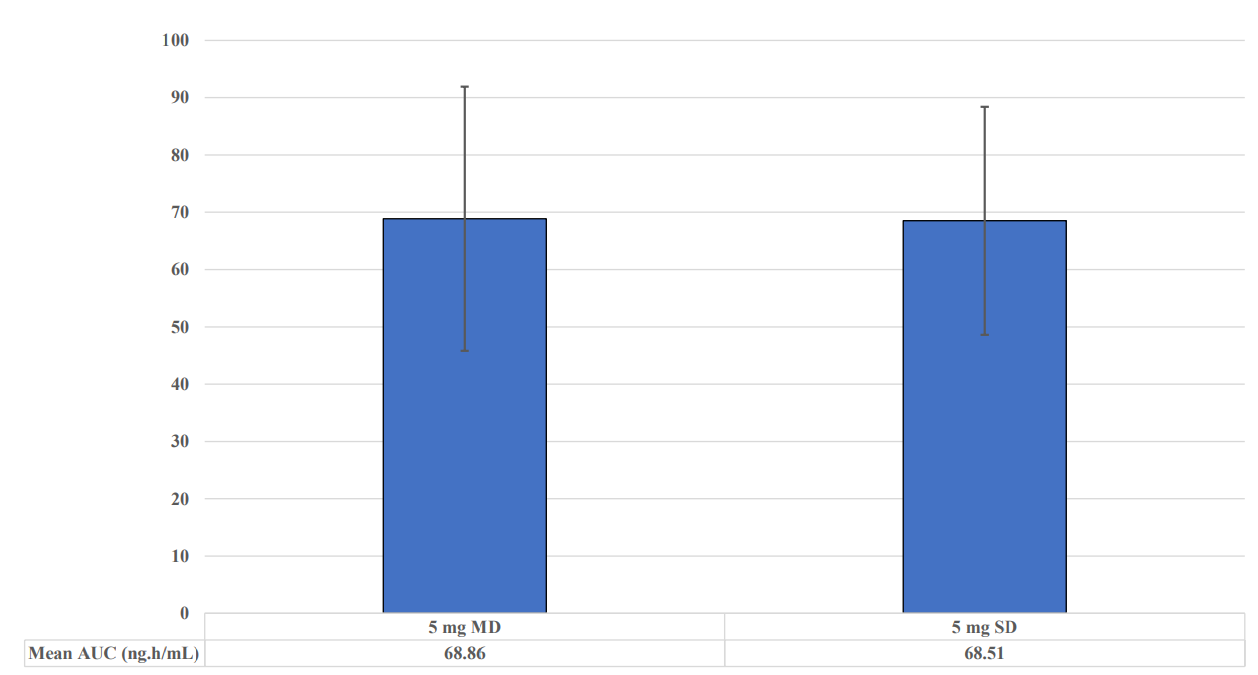
• Cmax and AUC of oxycodone were comparable following single-dose and multiple-dose for both 80 mg and 5 mg strengths respectively
• Comparable variability of PK parameters observed from single-dose and multiple-dose trials when given either at 80 mg or 5 mg; demonstrated in Figures 1 and 2 below