Clinical Research Spotlight: Ketamine, Norketamine and Hydroxynorketamine
Ketamine is a precipitation drug that was developed in the 1960s and approved as an anesthetic. Ketamine has been used on the battlefield to treat injured soldiers in the Vietnam War, in pediatric sedation, and is a medicine widely used in veterinary anesthesia. In addition to its beneficial pharmacological action, Ketamine also produces dissociative and hallucinogenic effects, and consequently is considered a psychedelic and has been branded a “party drug”.
Due to Ketamine’s high abuse-potential, it saw limited clinical use until the 1990s, when it was demonstrated that at sub-anaesthetic doses, in addition to the profound psychedelic effects, it possessed antidepressant activity.
Major depressive disorder occurs in about 16% of the population in their lifetime. The onset of action of commonly-used antidepressants (e.g. fluoxetine, sertraline and citalopram) is very slow, requiring daily drug administration over weeks or months, with some patients still failing to respond to treatment. In the past 15 years, there have been a dramatic surge in clinical studies on the “off-label” use of Ketamine in the treatment of depression and suicidal behavior.
Ketamine works quickly, is safe, and has the advantage over other psychedelics in that it is legal. As a consequence, Ketamine-assisted psychotherapy (KPT) has gone mainstream with more than 300 ketamine clinics in the US today. Due to its rapid and short-lived onset, ketamine is considered by many as the 1st novel anti-depressant in a decade for patients who have exhausted every other avenue of treatment. Finally, a broader clinical use of Ketamine-induced neuroplasticity has been indicated in other central nervous system (CNS) diseases such as Alzheimer’s and Parkinson’s.
BioPharma Services has the experience and expertise in designing a ketamine bioanalytical method, which requires knowledge in pharmacology, workings of the central nervous system, and the potential targets of the drug under study.
Metabolism and Pharmacokinetics
Ketamine is extensively and stereoselectivity metabolized by multiple hepatic cytochrome P450 isoforms into metabolites, including N-Desmethyl ketamine (norketamine), Dehydro norketamine, Hydroxynorketamine and Deschloroketamine. Most of the early research studies had the commonly accepted view that Ketamine and its metabolite norketamine were the pharmacologically active forms, and this is where the term the “ketamine paradigm” originated.
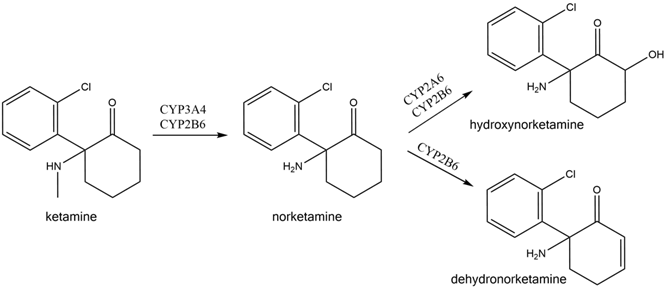
Based on this hypothesis, the other metabolites were considered inactive, and their pharmacological properties were not assessed further. In more recent years, however, data from in vitro and in vivo studies have demonstrated that another metabolite, hydroxynorketamine, has potent pharmacological effects and is responsible for the anti-depressant effects of Ketamine and formed the basis of the newly coined term the “ketamine metabolite paradigm”.
Development of a Bioanalytical Method (Ketamine and Norketamine)
For application in clinical investigations of the “ketamine paradigm” for anesthetic indications of ketamine, a bioanalytical method has been successfully developed at BioPharma Services. The bioanalytical method covers a calibration range of 0.40 to 100.00 ng/mL for Ketamine and 4.00 to 1000.00 ng/mL for Norketamine.
The developed bioanalytical method utilizes a protein precipitation extraction procedure requiring 50 µL of biological matrix (human plasma) spiked with stable labeled internal standards (Ketamine-D4 and Norketamine-D4). Extracted samples are chromatographically separated on a C18 HPLC column using a gradient with Mobile Phase A (5.0 mM ammonium acetate) and Mobile Phase B (100% acetonitrile). The liquid chromatography mass spectrometer (AB Sciex QTRAP 5500) is operated in positive ion mode using a turbo Ion spray source and MRM detection. The total chromatographic run time for a 5.0 µL injection is 5.0 minutes per sample.
Development of a Bioanalytical Method (Hydoxynorketamine)
For application in clinical investigation of the “ketamine metabolite paradigm” for antidepressant indications of ketamine, a bioanalytical method has been successfully developed at BioPharma Services. The method covers the calibration range of 0.25 to 50.00 ng/mL for hydroxynorketamine
The developed bioanalytical method utilizes a supported liquid extraction procedure requiring 100 µL of biological matrix (human plasma) spiked with stable labeled internal standard (hydroxy norketamine-D6). Extracted samples are chromatographically separated on a biphenyl analytical HPLC column using a gradient with Mobile Phase A (5.0 mM ammonium trifluoroacetate) and Mobile Phase B (50/50 acetonitrile/methanol). The liquid chromatography mass spectrometer (AB Sciex QTRAP 5500) is operated in positive ion mode using a turbo Ion spray source and MRM detection. The total chromatographic run time for a 10.0 µL injection is 4.5 minutes per sample.
Summary
Under optimized extraction, chromatographic and liquid chromatography mass spectrometry detection conditions, selective, sensitive, accurate and precise bioanalytical methods were developed and validated for Ketamine, norketamine and hydroxynorketamine. The validated methods were free of matrix effect, and no impact from other main metabolites (Dehydro norketamine and Deschloroketamine) were found.
Ketamine, norketamine and hydroxynorketamine were found to be stable in biological matrix for 24 hours at room temperature/under chilled conditions, after 3 freeze and thaw cycles and after long-term frozen storage -20°C and -70°C .
The bioanalytical methods are suitable for application in the pharmacokinetics assessment of therapeutic doses of the novel drug ketamine, and its metabolites norketamine and hydroxynorketamine and in human clinical trials.
These methods add to the growing list of available bioanalytical methods at BioPharma Services suitable for application in the expanding field exploring the therapeutic benefits of psychedelic drugs in treating psychiatric disorders.
Find out why BioPharma might be the right partner for you! Learn more about BioPharma Services and the wide array of services we provide.
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials I/IIa and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.



