Reasons and Solutions for Multiple Peaking Phenomena in Pharmacokinetics Studies

In this blog, we provide a brief overview of the multiple peaks in pharmacokinetics studies, how to handle and the potential impact on study outcomes.
In the pharmacokinetics (pharmacokinetic) study, a multiple peaking phenomenon refers to the occurrence of more than one peak in the concentration-time profile of a drug in the circulation system after administration. In other words, instead of a single peak followed by a gradual decrease in drug concentration, the concentration-time curve displays two or more distinct peaks separated by lower concentration periods.
For example, the occurrence of multiple peaks of monomethyl fumarate was observed in an in-house study under fed conditions (Figure 1), resulting in higher variability for all pharmacokinetic parameters, and the AUCinf for some subjects cannot be estimated.
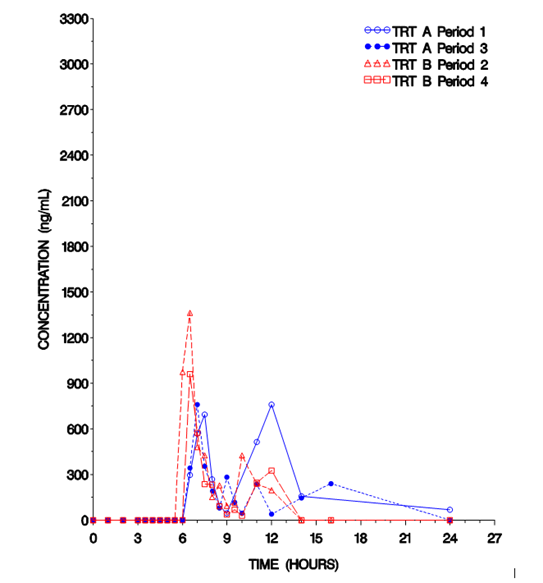
Figure 1. Typical Individual Plasma Monomethyl Fumarate Concentration under Fed conditions
Causes of Multiple Absorption Peaks
The presence of multiple peaks in drug concentration does not necessarily indicate a problem or adverse effect. It is drug’s pharmacokinetic parameter, which can be influenced by various factors such as:
-
- Absorption Mechanisms: If a drug is absorbed through different mechanisms or sites in the gastrointestinal tract, it might result in multiple peaks due to variations in absorption rates.
- Enterohepatic Circulation: Some drugs are excreted into the bile, reabsorbed in the intestines, and then re-enter the bloodstream. This process, known as enterohepatic circulation, can lead to secondary peaks in the concentration-time profile.
- Complex Metabolism: If a drug undergoes complex in vivo metabolism, including the formation of active metabolites with their own absorption and elimination profiles, multiple peaks can occur in the metabolites’ profiles.
- Distribution Effects: Drugs that distribute extensively to tissues and have varying degrees of tissue binding can exhibit multiple peaks as the drug redistributes between the bloodstream and tissues.
- Modified Release Formulations: Drugs formulated for delayed/sustained release or multiphasic release can result in a lag time before the drug is absorbed, leading to multiple peaking in the pharmacokinetic profile.
- Drug Interactions: Concurrent use of other drugs or substances that affect absorption, metabolism, or elimination can contribute to the occurrence of multiple peaks.
- Food Effect: Food intake and meal timing can influence drug absorption rates and might contribute to the occurrence of multiple peaks.
- Physiological Variability: Individual variability in absorption, metabolism, and elimination processes can result in multiple peaks, especially if different individuals metabolize the drug at different rates.
It is important to note that in the scope of this discussion, we will focus on the multiple peaking phenomenon occurred due to the drug characteristics and study conditions, which will likely to observed in the majority of populations.
Impact of Pharmacokinetic Parameters and Bioequivalence Estimation
It’s important to note that the interpretation and significance of the AUCinf value in the context of multiple peaks may vary depending on the specific drug and its pharmacokinetic parameters. The AUCinf is typically used to assess the overall exposure to a drug and can provide important information about the drug’s efficacy and safety profile.
In the context of bioequivalence studies, the AUCinf is one of the key pharmacokinetic parameters evaluated to compare the exposure profiles of different formulations. Comparing the AUCinf values of the test and reference formulations helps determine if they are essentially the same or if there are significant differences in their overall exposure. Statistical analysis is performed to assess the similarity of the AUCinf values, considering any variations in multiple peaking patterns.
Regulatory agencies, such as the FDA, EMA, and NMPA, have specific criteria for bioequivalence assessment, including acceptance ranges for AUCt, AUCinf and Cmax. The multiple peaking might lead to deviations from these criteria, and regulatory authorities may require additional analyses or explanations to demonstrate that the products are indeed bioequivalent.
For FDA submission, AUCinf is one of the key pharmacokinetic parameters to establish bioequivalence, therefore when multiple AUCinf cannot be estimated, it may fail the study.
For other agencies such as EMA/TPD/NMPA/Health Canada, the acceptance criteria for bioequivalence generally apply for only AUCt and Cmax, thus, the multiple peaking phenomenon might have less impact on the study outcome.
The specific regulatory guidelines and requirements for bioequivalence assessment may vary among different regions or authorities. It is important to consult the relevant guidelines and work with regulatory agencies or experts in the field to determine the appropriate approach when dealing with complex concentration-time profiles and the inability to estimate the elimination phase pharmacokinetic parameters such as lambda, half-life and AUCinf accurately.
For Phase 1 clinical trials, if multiple peaking is observed, further investigations may be needed to characterize the drug’s pharmacokinetic parameters more thoroughly and to optimize dosing regimens in late phase. This may involve conducting more extensive pharmacokinetic studies, including phase II and phase III trials, to refine the drug’s administration and dosing recommendations.
How to Minimize the Impact of Multiple Absorption Peaks
The presence of multiple peaks could raise questions about the clinical implications of these absorption differences in statistical significance and clinical relevance. It is important to assess whether these differences are likely to impact the drug’s safety and efficacy in a meaningful way.
Multiple absorption peaks are often unavoidable due to a variety of reasons, but there are a few measures that can be taken to minimize the impact of multiple absorption peaks on experimental results.
-
- Optimize sampling timepoints, when designing bioequivalence studies involving multiple peaking drugs, researchers need to carefully plan the study design, the choice of sampling time points in a bioequivalence study becomes critical when dealing with multiple peaks.
- To increase sample size and power, bioequivalence studies rely on demonstrating that the 90% confidence intervals for the ratio of geometric means of Cmax and AUC fall within a predefined acceptance range. ultiple peaking can lead to higher variations in these pharmacokinetic parameters and potentially affect the assessment of equivalence. Depending on the extent of variability caused by multiple peaks, increase sample sizes or statistical power, apply reference-scaled bioequivalence approach are necessary to ensure the study pass.
- Formulation Development: For drug development, understanding the reasons behind multiple peaks can guide formulation strategies to optimize drug release and absorption profiles.
- Modeling and Simulation: Mathematical modeling and simulation are commonly used to predict drug behavior in the body. When multiple peaks are present, more sophisticated models may be needed to accurately describe the pharmacokinetic profile and predict drug exposure over time.
Why Choose BioPharma Services for Your Next Drug Development Project?
The multiple peaking phenomenon in pharmacokinetic studies is a complex issue that can arise due to a myriad of reasons, from absorption mechanisms to physiological variability. While it doesn’t necessarily indicate a problem, it does require careful consideration and understanding, ensuring accurate interpretation of study results. The presence of multiple peaks can influence key pharmacokinetic parameters, potentially impacting bioequivalence estimations and the overall understanding of a drug’s behavior in the body.
Addressing the multiple peaking phenomenon requires a multifaceted approach. Optimizing sampling time points, increasing sample size, refining formulation strategies, and employing advanced modeling and simulation techniques are just a few of the strategies that can be employed to navigate the challenges posed by multiple peaking.
Choosing a partner like BioPharma Services for your drug development process ensures that you are working with experts who are well-versed in these challenges. Our team is equipped with the knowledge, experience, and tools to handle the intricacies of multiple peaking phenomenon, ensuring that your drug development process is both efficient and effective.
With our expertise in pharmacokinetics and a deep understanding of regulatory requirements, we can guide you through the complexities of drug development, ensuring that your product meets the highest standards of safety and efficacy. If you’re looking for a partner who can navigate the challenges of multiple peaking phenomenonand deliver reliable, actionable insights, BioPharma Services is the clear choice.
Written By: David Yu, Pharmacokinetic Associate.



