Drug-Drug Interactions in Trial Research
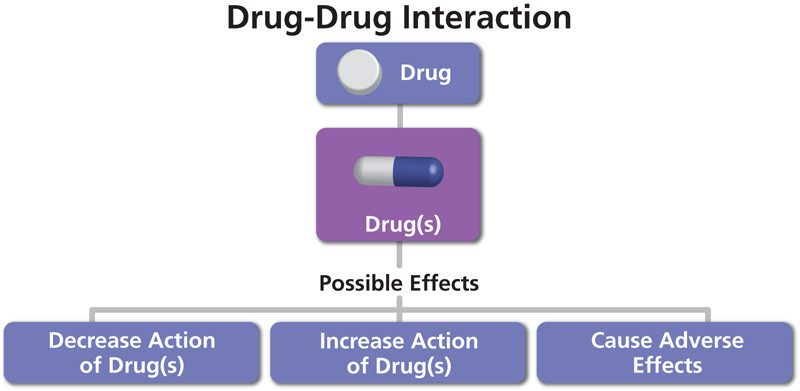
A drug-drug interaction (DDI) is a reaction between two (or more) drugs. A DDI can have pharmacology effect and Pharmacokinetic (PK) effect. The pharmacological effect of one or both drugs may be increased or decreased, or a new and unanticipated adverse effect may be produced, and therefore minimizing the drug interaction during the patient therapy is critical.
Drug-drug interactions may result from pharmacokinetic interactions (absorption, distribution, metabolism, and excretion) or from interactions at drug receptors. Interactions during drug absorption may alter the amount of drug absorbed and change therapeutic effectiveness. Drug interactions in patients receiving multiple drug regimens are a constant concern for patient treatment.
With the increased bioavailability of new drugs and their concomitant use with other drugs, there has been a rise in the potential for adverse drug interactions, as this has been demonstrated by the recent withdrawals of newly marketed drugs because of unacceptable interaction profiles. Therefore, the interaction potential of a new compound has to be assessed in detail, starting with preclinical in vitro and in vivo studies for candidate selection and continuously followed up through preclinical and clinical development.
Drug-Drug interactions with the CPY450 metabolism.
Many drug interactions are the result of an alteration of CYP450 metabolism. Cytochrome P450 (CYP450) enzymes are necessary for the detoxification of foreign chemicals and the metabolism of drugs. There are more than 50 CYP450 isoenzymes, but the CYP1A2, CYP2C9, CYP2C19, CYP2D6, CYP3A4, and CYP3A5 enzymes metabolize 90 percent of drugs.
These enzymes are predominantly expressed in the liver, although they also occur in the small intestine, lungs, placenta, and kidneys. The two most significant enzymes being CYP3A4 and CYP2D6. Genetic variability (polymorphism) in these enzymes can influence a patient’s response to commonly prescribed drug classes, with many beta blockers and antidepressants being part of the classes.
CYP 450 enzymes can be inhibited or induced by drugs, resulting in clinically significant drug-drug interactions that can cause unanticipated adverse reactions or therapeutic failures. For example, interactions with warfarin, antidepressants, antiepileptic drugs, and statins often involve the CYP 450 enzymes. About 7% of the white population and 2 – 7% of black people are poor metabolizers of drugs dependent on CYP2D6, which metabolizes many beta blockers, antidepressants, and opioids. One in five Asian persons is a poor metabolizer of drugs dependent on CYP2C19, which metabolizes phenytoin, phenobarbital, omeprazole (Prilosec), and other drugs.
Variance in drug response among populations of different ethnic origins also can be caused by genetic variations in other drug-metabolizing enzymes, drug transporters, and drug receptors.
Inhibitors block the metabolic activity of one or more CYP450 enzymes. The extent to which an inhibitor affects the metabolism of a drug depends upon factors such as the dose and the ability of the inhibitor to bind to the enzyme. For instance, sertraline (Zoloft) is considered a mild inhibitor of CYP2D6 at a dose of 50 mg, but if the dose is increased to 200 mg, it becomes a potent inhibitor. 1 Inhibitory effects usually occur immediately.
Inducers increase CYP450 enzyme activity by increasing enzyme synthesis and producing quicker metabolism of the drug taken. A decrease in the concentration of a drug metabolized by CYP2C9 can occur within 24 hours after the initiation of rifampin (Rifadin), an inducer with a short half-life, but can occur up to one week after the initiation of phenobarbital, an inducer with a very long half-life.
A drug also may be metabolized by the same CYP450 enzyme that it induces. Carbamazepine (Tegretol), a potent enzyme inducer, must be initiated at a low dose and then increased at weekly intervals as its half-life gradually decreases over time.
Since drug-drug interactions can really lead to the unwanted adverse event or treatment failure. The information of drugs metabolized by cytochrome P450 enzymes, as well as the most potent inhibiting and inducing drugs, are very critical to help minimize the possibility of adverse drug reactions and interactions for a potentially developed new drug.
FDA Guidance with drug-drug interactions
Food and Drug Administration (FDA) drug interaction guidance highlights the methodologies and criteria to guide the drug interaction evaluation by sponsor and regulatory agencies and to build up the informative product labelling for health practitioners and patients.
FDA has continued efforts to evaluate methodologies to study drug interactions and communicate recommendations regarding the conduct of drug interaction studies, particularly for CYP-based and transporter-based drug interactions, to the pharmaceutical industry. A drug interaction Website was established to document the FDA’s current understanding of drug interactions. [JH1]
Drug-drug interaction studies within the new drug development process (NDA)
During the new drug development process, drug-drug interactions studies are mainly to obtain the critical information to enable the regulatory decision-making regarding a new drug.
There are different types of drug-drug interactions studies, depending on the stage of the drug development. The DDI studies can be conducted both during the nonclinical phase and the clinical phase, here being the focus on the clinical phase of studies. Most of the drug-drug interactions studies are conducted with healthy volunteers in Phase 1 clinical trials, while some can also be conducted in target patient populations at Phase 2 or 3 stages. Earlier DDI information at the development phase can help to plan the further clinical trials.
The DDI study will assess the potential drug-drug interactions between a new drug and a number of inhibitors or inducers of metabolic enzymes. The well-known enzymatic inhibitors or substrates are co-administered with the investigational drug to assess the potential interaction and in most cases the worst-case scenarios to be designed within the human clinical trial. If the PK of an investigational drug is not altered when administered concomitantly with a probe inhibitor, the newly developed drug should not be expected to be involved in these types of drug-drug interactions
Pharmacokinetics drug-drug interaction
Theoretically, drug-drug interactions compares drug substrate (D) concentrations with and without the interacting index drug (I) for PK interaction. The trial can be:
- A parallel design (D in one group and D + I in another group)
- A conventional crossover design (with 2 sequences of D followed by D+I and D+I followed by D)
- A one-sequence crossover (D always followed by D + I or the reverse).
The study drug dose selection should be based on the factors such as: the acute or chronic use, safety aspects, PK even PD properties of the study drug and probes. The DDI trials can also be done in both single and multiple doses. The inhibiting/inducing probe drugs and the drug substrates should be dosed so that the exposures to both drugs are appropriate to their clinical use for healthy subjects, when possible, including the highest doses likely to be used clinically.
Beside the drug-drug interactions studies for NCE development, there are also new drug formulations modified or combo drugs from the existing compounds via the 505(b)(2) submission route. The DDI studies for such new drug formulation will focus on if there are any alterations of the PK of two or three compound(s) when giving as the new formulation comparing to the standalone compound instead of the emphasis of the enzymatic probes.
There are many known expected co-administration medications or modified release formulations that require the DDI studies. Such DDI study usually a crossover design (can be more than 2 arms) with one arm as the new combo formulation while the other arms as the standalone drug administration to assess if there are any PK changes.
In recent years, the drug-drug interactions could also be predicted using modeling such as Physiologically based pharmacokinetic (PBPK) models are the approach to assess the potential DDIs. Such model considers the impact of concomitant drugs and utilizes human physiology, enzyme, and transporter abundance data to construct the model for prediction of the DDI.
BioPharma’s Drug-Drug Interaction Study Services
Evaluating the drug-drug interactions is essential to drug development, since most patients take more than one medication simultaneously. More people are interacting with multiple drugs at one time, the drug absorption may therefore alter the amount of drug absorbed into the body and change the overall therapeutic effectiveness. BioPharma Services has experience designing and analyzing DDI studies at phases of drug development. Contact us for tailored scientific advice on your DDI study and how it can impact a future trial you may be running.
Find out why BioPharma might be the right partner for you! Learn more about BioPharma Services and the wide array of bioanalytical services we provide.
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials 1/2a and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.



