Exploring the importance and ways to achieve Steady State in Pharmacokinetics

An Introduction to Multiple-dose Study
Dose and dose regimen are some of the features that need to be determined first when designing a clinical trial for an investigational product (IP). While providing a single dose of study drug is often facilitated to quickly assess the safety, tolerable and Pharmacokinetics (PK) of the IP at the early phase (Phase 1 and 2a), and to evaluate comparable bioavailability/ bioequivalence (BE) at later phase; the timing of a multiple-dose study occurs during any stage of a clinical trial, including but not limited to early-stage development to evaluate the safety, tolerability, and the pharmacokinetics of the new drug in healthy volunteers; and late-stage development to assess the safety, efficacy, and PK of the product in patients. It is also a part of BE studies required for some special cases that determines whether two tested formulations are similar in bioavailability after multiple-dose administration.
A Pharmacokinetics multiple-dose study, or repeated-dose study, is where subjects receive repeated drug administration with the same dose or different dose levels for a defined duration to assess the Pharmacokinetic properties of the test drug product. A multiple-dose study typically advances from single-dose studies to further investigate the PK properties of the drug over an extended period of time. A multiple-dose study can be called as Steady state study, if the dose regimen allows drug concentrations built-up to reach the steady state conditions.
Understanding Steady State: What Is It, and What Determines It?
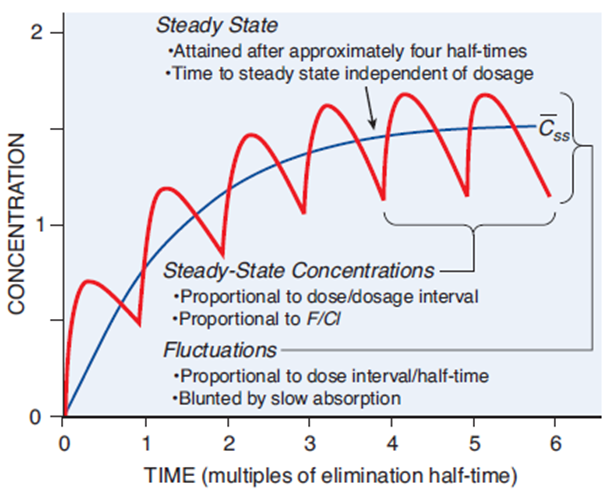
When drugs are given in the same dose, dosing interval, and tend to build up in the body fluids over time (i.e., accumulation potential), it reaches a plateau concentration called steady state. This is when the rate of administration is equivalent to the rate of drug elimination, and hence drug concentrations remain within a stable window for a consistent period. For the ultimate goal of clinical trials, this window should be the same or contain within the targeted therapeutic index (refer to Figure 1).
The time to achieve a steady state is dictated by individual characteristics (e.g., age, body weight, hepatic function), the drugs’ half lives and dosing regimen (dose, frequency, and duration). It is important to note that changing the dose or dosing interval might not accelerate the time to attain steady state, but it may increase or decrease the blood concentrations at stable levels. Majority of drugs takes approximately five to six half-lives to reach steady-state, which are crucial factors that determine the dosing duration and washout period of a multiple-dose study in BE.
Figure 1 – Fundamental pharmacokinetic relationships for repeated administration of drugs
What Pharmacokinetics Parameter is Used to Assess Drug Accumulation and, What Does it Implicate?
The accumulation index (AI), accumulation ratio/rate (AR), or ratio of accumulation (RA) is a parameter that assesses the extent of drug accumulation after multiple-dose administration. It is defined as the ratio of rate and extent of absorption at steady state as compared to that after the first dose, where the value of 1 means no accumulation.
Drugs with high AI/AR/RA implicates a lower dose and/or longer dosing regimen might be required to achieve therapeutic concentrations without increasing the risk of toxicity. It is noteworthy to point out that high AI/AR/RA should not simply be interpreted as high toxicity risk, as the ratio is impacted by the dosing frequency. Alternately, drugs with low AI/AR/RA implies the drug is eliminated in the body quickly and hence a higher dose or a shorter dosing regimen might be adjusted to better maintain the therapeutic concentrations.
Achieving Steady State Could Be More Challenging Than One Thinks.
Although the ideal goal to optimize therapeutic effects is to maintain drug levels at steady state, not all drugs could yield stable concentrations. For instance, drugs with flip-flop kinetics can not achieve a true steady-state concentration, as their absorption is considerably slower than elimination. Consequently, the plateau concentration is lower than the theoretical concentration, as the quick elimination would hinder a complete absorption process of the drug that could have provided a higher therapeutic level. Hence, clinicians evaluate the dosing regimen based on the target peak concentrations rather than steady-state levels for these drugs.
Despite most drugs attaining plateau blood levels by providing repeated dose as described above, some drugs require alternative ways to achieve steady state. First, for drugs that have a slow onset of action or typically long half-lives but require reaching desired therapeutic effects quickly, a loading dose could be given in intravenous infusion to rapidly yield therapeutic level and followed by a lower maintenance dose to maintain a stable therapeutic concentration. For instance, phenytoin is commonly initiated with a loading dose to promptly control seizures and proceeds with small increments in dosage to adjust the therapeutic levels based on symptom severity.
Second, for drugs that have severe side effects, narrow therapeutic index, or different dosage throughout disease progression, a titrated dose could be administered to manage the safety and undesired adverse events. This is usually followed by a step dose to closely monitor the side effects and efficacy prior to adding the maintenance dose for optimized and stable therapeutic response. Antidepressants and antipsychotics are often prescribed in the lowest effective dose to provide clinical effects while minimizing unwanted adverse reactions.
It is worth mentioning that while many are familiar with up-titration, down- or cross-titration are sometimes neglected. In real-life, a physician might need to discontinue or switch from one treatment to another due to the patient’s response or drug supply shortage, while in fact many drugs should not be discontinued abruptly. For example, the dose of opioids is titrated down until complete discontinuation due to withdraw symptoms and risk of addiction. Switching between antipsychotic drugs is also common practice that mostly requires cross-titration, when the dose of first drug is gradually reduced, and the dose of second drug is gradually increased.
When developing a new drug, performing various multiple-dose studies with different dose regimen are crucial and unpreventable to the understanding of drug properties as it reflects real-world human exposure to drugs that are often prescribed for a short or prolonged duration. Not only can clinicians then determine the appropriate dose and regimen to ensure therapeutic drug concentrations are maintained in patients through a multiple-dose study, but it can also help to predict drug-drug interaction in patients on multiple medications.
This is especially important for drugs with long half-lives or narrow therapeutic index, where it is critical to identify the accumulation risk in order to avoid unexpected side effects and achieve therapeutic exposure. However, with a good development drug planning, thorough study designs and learning from the obtained data, the pipeline time can be significantly reduced.
Regulatory Requirement and Special Approach in Multiple-dose Study for Bioequivalence Purpose
For generic product, regulatory agencies have different requirements on performing multiple-dose study.
For drugs with immediate-release dosage form, multiple-dose study is generally not required as they have a quick onset of action, and their Pharmacokinetic properties are established.
For drugs with modified-release dosage form, FDA does not require steady-state studies unless warranted by some circumstance that a single-dose study in a healthy volunteer is not sufficient, such as cytotoxic drugs that would require a multiple-dose, clinical-endpoint study in patients.
Similar to FDA, TPD also does not require multiple-dose study, except for some critical dose drugs. Whereas EMA has a distinct guideline where steady-state studies should be conducted when the test product demonstrates a risk of accumulation (More details can be accessed in EMA Guideline on the PK and clinical evaluation of modified release dosage forms – EMA/CHMP/EWP/280/96 Rev1).
One interesting factor in designing multiple-dose BE studies is that a strategic approach can be taken advantage of for highly variable drugs. While single dose studies using the widening BE approach for highly variable drugs requires a replicated treatment dosing in two separate periods, steady-state studies could utilize the repeated dosing in one period to employ the widening BE approach.
To complete, the Pharmacokinetic sampling data could be collected on two consecutive dosing intervals after reaching steady-state to estimate the intra-subject variability of the drug product. Hence, this provides a cost-effective way to optimize the study design for a highly variable drug that has a risk of accumulation.
Why Choose BioPharma Services?
Multiple dose studies provide valuable Pharmacokinetics data after repeated administration that would help to guide the dosing regimen and minimize the safety risk to patients. Not only is it essential to new clinical drug development, but also could be an important part of generic drug submission in some circumstances. A deeper navigation into the regulatory guidelines could strategically facilitate the clinical drug research.
BioPharma Services has extensive experiences in executing multiple-dose study from scientific design to complete clinical study report for submission stage. We have performed more than 20 successful multiple-dose studies within the last 3 years, tailored to different regulatory agencies.
Written by: Jacinda Kwok
If you are interested in other steady-state study and would like to discuss further to customize a design for the objective, please visit our Contact page and an expert Pharmacometrics team member will be happy to discuss our capabilities with you. Find out why BioPharma might be the right partner for you! Learn more about BioPharma Services today.
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials 1/2a and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.



