Importance of Study Restriction: The Avoidance of Drug-Drug Interactions Part 1
Drug interaction management is a significant concern in designing clinical trials, especially those focusing on pharmacokinetic (PK) endpoints. Such interactions, both expected or unexpected drug-drug interactions, may arise from the prescribed treatment regimen, emerging therapy for side effects, or participants’ lifestyle which can interfere with the study evaluation and threaten participants’ safety
While these interaction’s are inevitable in clinical trials, proactive identification of potential interactions and the establishment of appropriate study restriction protocols during protocol development can standardize procedures, enhance participant compliance, and safeguard the integrity of data.
This blog will explore several common study restrictions typically included in protocols targeting drug-drug interactions, alongside Biopharma Services (BPSI) recommendations best practices for drug interactions analysis and management.
1.1 Cytochrome P450 (CYP) modifiers or competitive substrates
1.1.1 Mechanism
There are at least 57 human CYP genes and their respective enzymes, however, only a small number of CYP isoforms (CYP1A2, 2A6, 2B6, 2C8, 2C9, 2C18, 2C19, 2D6, 2E1, 3A4, 3A5, and 3A7) participate in the metabolism of drugs. It’s estimated that nearly 75% of all drugs undergo CYP enzyme oxidative metabolism and ~50% of those are at least partially affected by CYP3A4.
There are two different ways for drug interactions with CYP, one way is inducing the CYP enzymes and the other way is inhibition. Firstly, for induction of CYP to occur, a drug should bind to a receptor, which serves as an up-regulator of CYP gene expression. Therefore, the signaling cascade will turn on and produce more CYP enzymes which further enhances the drug metabolism.
On the other hand, inhibition of CYP is simpler as it mostly results from the direct interaction of the antagonist with the enzyme, rather than from a decrease in the expression of CYP proteins.. The majority of the direct interactions are either reversible allosteric binding, irreversible binding, or competitive substrates due to multiple binding regions or sites on CYP enzymes.
Any form of modification on CYP will pose risks during a clinical study as it interacts with the investigational product (IP) by either decreasing or boosting the exposure. Table 3 is a list of examples of CYP enzyme substrates and inhibitors.
1.1.2 Example
Both rifampin and phenytoin are examples of CYP 3A4 inducers and exert the effect by binding onto the pregnant X receptor (PXR) which upregulates CYP 3A4 gene expression. Nevertheless, the PXR gene also regulates other genes such as MDR1 encoded for transporters and efflux protein. Therefore, dosing an IP that metabolizes via CYP 3A4 with rifampin or phenytoin will cause a drastic reduction in exposure and affect the pharmacokinetics of the IP.
In other examples like terfenadine, it would be a competitive substrate on CYP3A4. In such cases, a stronger substrate on CYP3A4 will increase the exposure of terfenadine (increased AUC and Cmax) and lead to associated toxicity such as cardiac arrhythmias.
Lastly, take diltiazem as an example, it will bind to CYP3A4 and the binding will be irreversible and lead to a permanent inactivation of CYP. Such inhibition will require the replenishment of the enzyme, with the restoration of enzymatic activity based on the rate of synthesis for new enzymes.
1.1.3 Restriction recommendation
In general, there are various ways to modify CYP enzymes and to take care of the drug interaction management. Taking the irreversible bindings into account, usually 30 days of restricted use of CYP modifiers prior to dosing and until the end of the study would be considered sufficient to minimize the effect from previous use of any CYP modifiers.
1.2 Hormonal Contraceptives
1.2.1 Mechanism
CYP P450 series enzyme in the liver was the major metabolic pathway for orally administered hormonal contraceptives. Most of the contraceptive steroids available on the market were metabolized via CYP3A4, one of the isoforms of the CYP P450. CYP3A4 is a major cytochrome P450 which catalyzes a broad range of substrates including procarcinogens and ~50% of all active pharmaceutical ingredients. During the study design, an evaluation on the allowance of hormonal contraceptive use is needed. One of the major reasons is to minimize the potential drug-drug interaction between our investigational product (IP) and the hormonal contraceptive.
Table 4 shows all the hormonal contraceptives either inhibiting CYP3A4 or being a substrate with CYP3A4. Therefore, the metabolic pathway of our IP will need to be carefully evaluated. Otherwise, there are risks to the study participants which reduce the efficacy of hormonal contraceptive and/or increase the chances of adverse effects by increasing the exposure to the investigational product.3,4
1.2.2 Case example
Taking ritonavir as an example, it acts as an inhibitor towards cytochrome P450 3A. When designing a clinical study for ritonavir, a restriction on the use of hormonal contraceptives is recommended to provide sufficient protection for the study participant. When using ethinyl estradiol in combination with ritonavir, the Cmax and AUC of ethinyl estradiol decreased by 32% and 44% respectively.3 It indicated a reduced effect of contraception and would lead to unexpected pregnancy. Also, a life-threatening adverse event could be a possible outcome with increased exposure to ritonavir as a result of a combination use of hormonal contraceptives and ritonavir.
However, taking another anti-viral agent emtricitabine as an example, we see that Emtricitabine has a minimal liver metabolism and is excreted mostly unchanged in urine.3 In this case, the restriction of the use of hormonal contraception may be unnecessary since it is expected to have minimal interactions.
1.2.3 Restriction recommendation:
If the IP is known to metabolize via CYP3A or cause any modification such as inducing or inhibiting CYP3A, we recommended prohibiting the use of any forms of hormonal contraceptive including vaginal ring, implant, and injection during the study to prevent any ineffective birth control nor increase exposure to the IP, albeit a cross-over study design may not cause a significant impact to the results due to the intra-subject comparison on different periods.
Considering the mean half-life of various contraceptive methods, a restriction with 6 months of restricted use of any hormonal contraceptive method prior to dosing and until the end of the study would be considered sufficient. In the meantime, participants can pursue non-hormonal intrauterine devices, or double barrier methods (eg: vaginal spermicidal suppository with male condom) when participating in studies that have restrictions on hormonal contraceptives.
1.3 Smoking
1.3.1 Mechanism
Compared to hormonal contraceptive which affects CYP3A4 the most, cigarette smoke affects other CYP isoforms. There are 4000 chemical compounds that could be found in cigarette smoke where some constituents have shown to either stimulate or induce CYPs. The polycyclic aromatic hydrocarbons (PAH) in cigarette smoking are responsible for the induction of CYP, especially CYP1A1, CYP1A2, and CYP2E1.
1.3.2 Case Example
In general, patients who are smokers may require a higher dose of drugs which are CYP1A2 substrates. However, if the patient decided to quit smoking during the treatment, CYP1A2 activity may be reduced over several days where serum or plasma concentration (Cmax) may increase and enhance the exposure of the active pharmaceutical ingredient towards the patient. Toxicity may occur due to the accumulation of the drug in a matter of days which could be dangerous. Take clozapine as an example, serum concentration (Cmax) of clozapine could rise 70% in 2 to 4 weeks after smoking cessation. A case reported a man stopped smoking while on treatment of clozapine which resulted in a clozapine-induced seizure. In which, the severity of interaction between smoking and pharmaceutical products could go beyond expectation.
1.3.3 Restriction recommendation
Light and heavy smokers can exert significant differences to the pharmacokinetic parameters such as Cmax and affect the study outcome. Thus, restriction of smoking is quite common for studies with a primary PK endpoint to minimize the potential interaction unless the investigation product (IP) is targeting for smoker population.
When the IP is primarily metabolizing via CYP1A2, “non-smokers only” is strongly recommended. This population is also recommended for clinical studies by both EMA and FDA (quit smoking for ≥12 months). In some cases, light-smokers (< 10 cigarettes per day) could be included if the investigational drug demonstrated a low drug-drug interaction potential with CYP1A2 agents.
1.4 Monoamine Oxidase Inhibitor (MAOI)
1.4.1 Mechanism
Unlike the above examples where the interactions are a pharmacokinetics mechanism, the interaction with MAOI has a pharmacodynamic mechanism. Monoamine oxidase inhibitors (MAOIs), bind onto the active site and inhibit the enzyme monoamine oxidase (MAO) which is crucial for the degradation process of various monoamines released by neurons and glial cells including dopamine, serotonin, and norepinephrine. Therefore, the intake of MAOI increases the exposure of dopamine, serotonin, and other neurotransmitters.
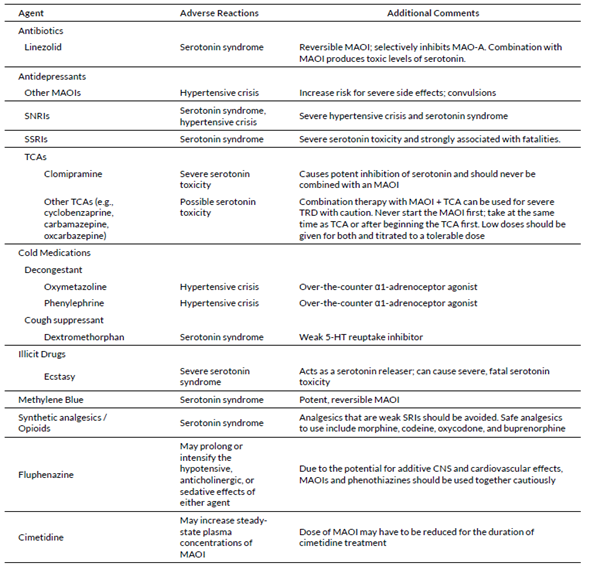
In usual synaptic cleft, neuron cells have several mechanisms to reuptake the excess neuron transmitters such as dopamine. However, when IP are sympathomimetic or serotonergic drugs, the use with MAOI could pose a life-threatening risk as they inhibit the reuptake mechanisms and in combination with MAOI, it can lead to synergistic effects and over-stimulate the neurons. Table 1 has a list of agents that could interact in case of concomitant use with MAOI.
1.4.2 Case Example
Tricyclic antidepressants (TCAs) exert the antidepressive effect by inhibiting the reuptake of serotonin, allowing it to remain in the synaptic cleft so it binds to the 5-HT receptor, thereby reducing depression symptoms.14 Natural MAO will break down the excessive serotonin to maintain a “safe” serotonin concentration. When TCA is used in combination with MAOI, the inhibition of serotonin reuptake by TCAs in addition to MAOI’s reduction of serotonin degradation, leads to serotonin accumulation in the brain, resulting in serotonin syndrome, a life-threatening adverse effect.
Similar effects can occur with other drug classes such as Serotonin reuptake inhibitors (SSRIs) and selective norepinephrine inhibitors (SNRIs). It’s important to note that, such compounds can be readily found in over-the-counter (OTC) cold remedies, emphasizing the importance of implementing clear and precise study restrictions to ensure the safety of participants.
1.4.3 Restriction recommendation
MAOI follows a similar pattern to the CYP modifiers. Inhibition can occur in either reversible allosteric binding, irreversible binding, or competitive substrate pathways towards MAO. Irreversible MAOIs such as phenelzine, isocarboxazid, and tranylcypromine can maintain the effect for up to 2 to 3 weeks. In this case, it is recommended to restrain “at least 30 days without the use of MAOI” prior to the first dosing until the end of the study. This restriction minimizes the risk of serotonin syndrome when having an investigation product belonging to the psychiatry drug class.
Why chose BioPharma Services for your next Drug Development Project?
The above are the most general and common restrictions concerning drug-drug interaction that are anticipated and should be considered during the study design. These restrictions serve to standardize the study observations, allowing reliable and repeatable study data. Additionally, they play a crucial role in protecting the subjects’ safety by mitigating the risk associated with any potential drug-drug interactions.
Drug interactions are very important and more common than you think in clinical trials, not only with other medications, but dietary and lifestyle could also be concerns. Stay tuned for our next discussion focusing on the food-drug interaction and explore further how to better prevent such bias in your next study.
Choosing a partner like BioPharma Services for your drug development process ensures that you are working with experts who are well-versed in these challenges. Our team is equipped with the knowledge and experience to evaluate and predict possible interaction and, thus, to apply an applicable restriction or exclusion criteria.
With our expertise in pharmacokinetics and a thorough understanding of regulatory requirements, we can navigate you through the obstacles during drug development, ensuring your product meets the expected safety and efficacy requirements.
Tables
Table 1: Common drug-drug interaction causes and study restrictions
| Study restriction | Mechanism | Impact | Suggested Restriction |
| Strong CYP modifier |
Inducer or Inhibitor |
· Increase rate/exposure of the IP · Decrease rate/exposure of the IP |
30 days prior dosing |
| Hormonal contraceptive | CYP3A4 substrate |
· Ineffective birth control · Increase the rate and exposure of IP (competitive substrate) |
Not allows for IP with CYP3A4 modifiers or primary CYP3A4 substrate |
| Smoking | CYP1A2 inducer | · Decrease rate/exposure of IP |
Population use: 1) Non-smokers or ex-smokers (≥12 months) 2) Light smoker (< 10 cigarette daily) when necessary |
| MAOI | Inhibit degradation of monoamines | · Increase exposure to monoamines such as serotonin which lead to serotonin syndrome | 30 days prior dosing |


Table 4: : List of available hormonal contraceptive on the market and their liver metabolism
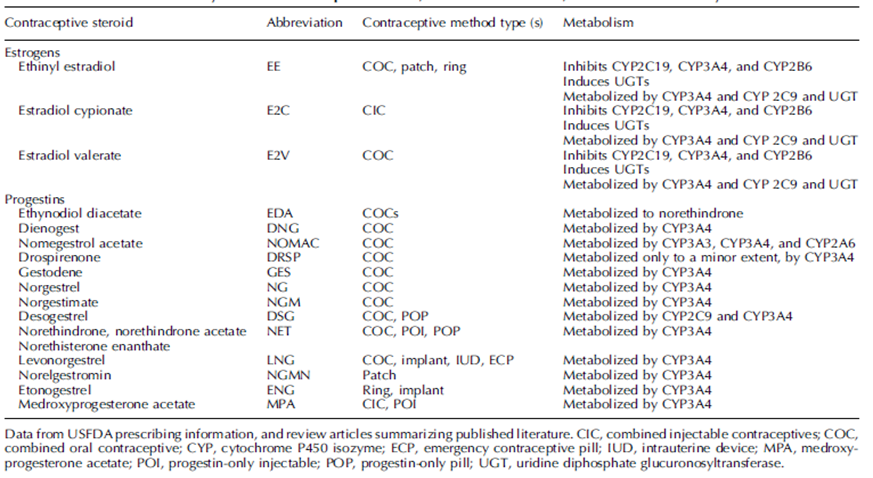
Table 5: Drugs that will interact when using with MAOI