


SPOTLIGHT ON: Ryan Best – Director of Clinical Research
SPOTLIGHT ON: Ryan Best – Director of Clinical Research My First Job I completed my Medical Laboratory Technician course in 2004, and like many others in my graduating class, I aspired to begin my career in a hospital setting. My work in the Clinical Research...
Writing Edit Checks in Clinical Data Management
Writing Edit Checks in Clinical Data Management Our Subject Volunteers are the most important part of our Clinical Trials and without them, there is no data. Without good, quality data, there is no accurate statistical analysis. So, how do we ensure the data we...
Surviving an FDA Inspection at a Clinical Research Organization (CRO)
Surviving an FDA Inspection at a Clinical Research Organization (CRO) This article is prepared to provide an overview of suggested steps to take prior to and during an FDA GCP Inspection of a Clinical Research Organization to achieve success. TYPE OF FDA INSPECTIONS...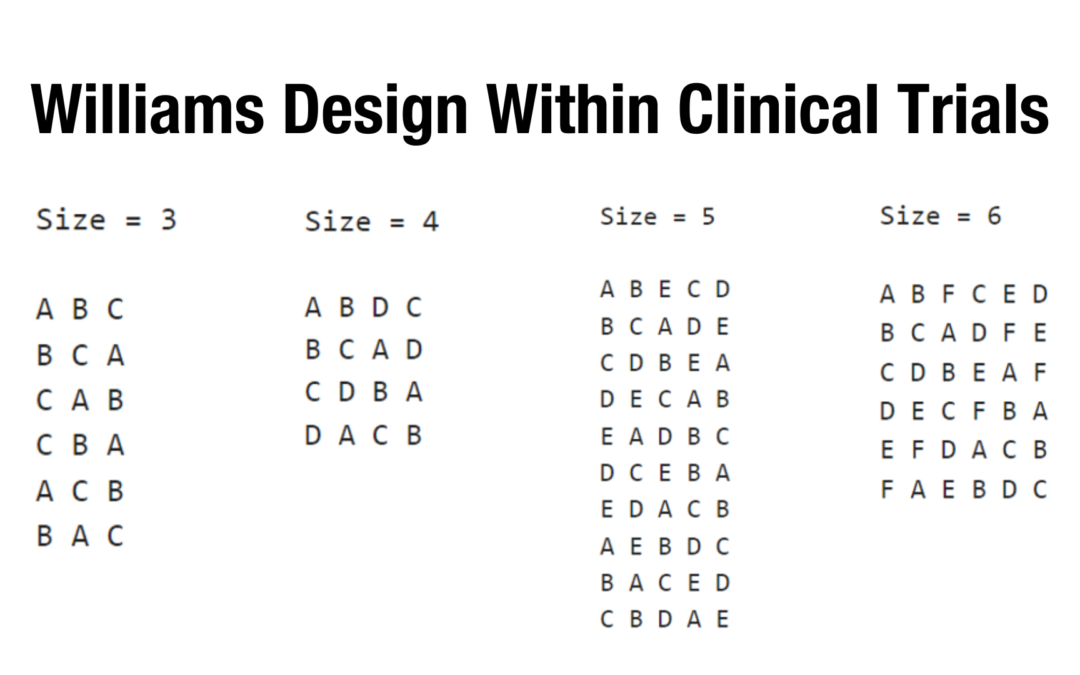
Williams Design and its Applications within Clinical Trials
Williams Design and its Applications within Clinical Trials Repeated measures design has been widely used in the early phase hap study, such as the two-way crossover design for a regular Bioequivalence study. Since the same subjects receive multiple measures, one...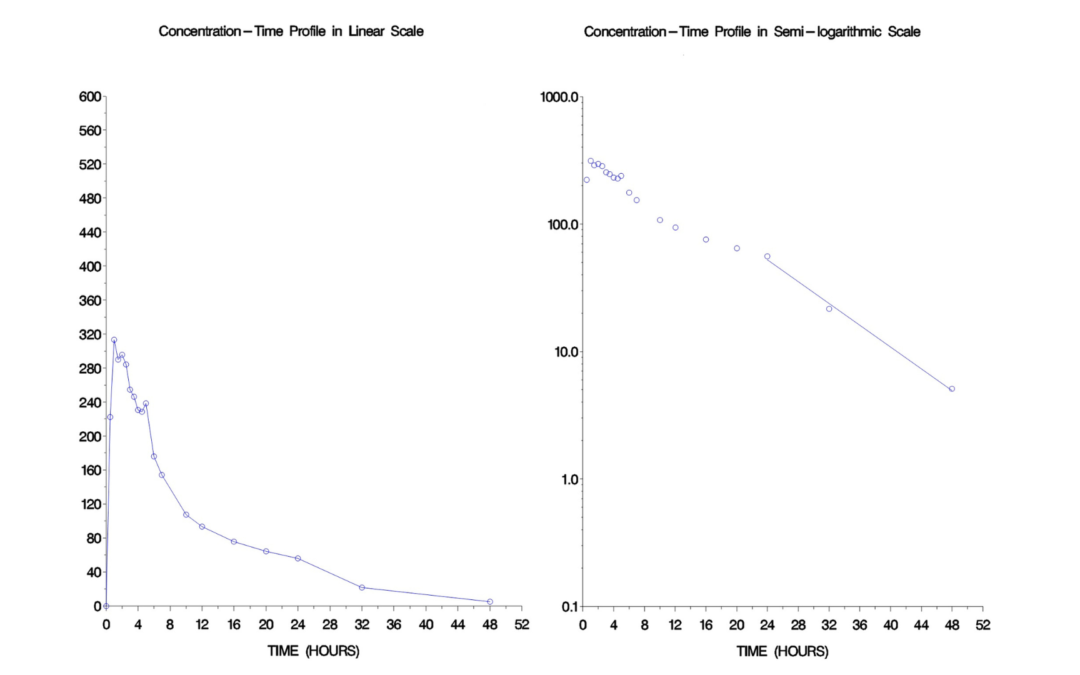

Recent Comments