Bioanalytical Lab: The Benefits of High Quality

The bioanalytical laboratory of BioPharma Services Inc. (BPSI) has a proven track record for scientific excellence, perseverance, and an unwavering commitment to provide services of the highest quality. This has allowed establishment of efficient laboratory workflows designed to reduce unnecessary repeat work/additional work.
This process starts at the bioanalytical method feasibility stage, where calibration ranges for a given dose of drug are optimized and the concentration of quality control (QC) samples are selected to be appropriate for the expected maximum concentration (Cmax) in clinical samples. For highly variable drugs, this frequently includes the selection of additional QC levels, to enable the presence of at least 2 QC levels covering study subject concentrations for all subjects.
As described in previous blogs, the metabolism of each drug is also carefully evaluated and any major metabolites (if commercially available) that would be present in clinical samples are included throughout both the method development and validation processes. This upfront work means that there are fewer surprises during the method application. BioPharma Service’s bioanalytical lab is proud of its track record for quality and routinely achieves batch passing rates of more than 95% and incurred sample reproducibility for methods of over 99% and frequently 100% rates are achieved. View all our Bioanalytical Assays here.
The developed framework, adhering to GLP certification standards and based on a commitment to high quality results in the field of bioanalysis, have several benefits.
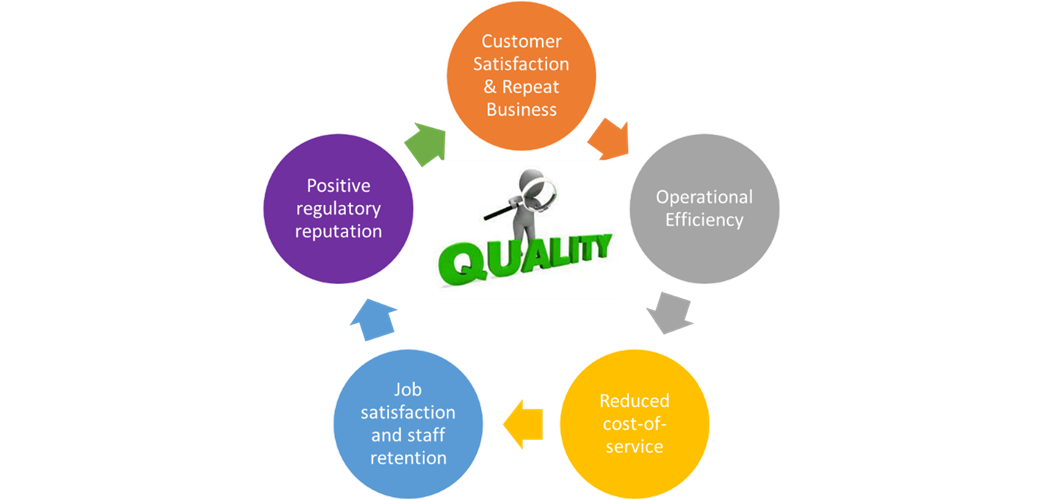
Customer Satisfaction
First and foremost, working with a lab focused on high quality, client’s trust the results. Having confidence in the data can have a direct impact on research outcomes. The timely receipt of data can also expedite the decision-making process in pharmaceutical research and development. A quality bioanalytical lab maintains open lines of communication with clients, keeping them informed of progress, potential issues and always armed with necessary solutions. Clear and transparent lines of communication from a lab means that clients are always aware of the status of their work, which helps in managing expectations and reducing uncertainty. Also, quality bioanalytical labs are open to customized approaches with tailored solutions to meet clients’ unique needs, including customized guidance on experimental designs, for example:
- Fit-for-purpose validation for challenging molecules. Validation approach customized for the intended use of the method.
- Client specific study plans using acceptance criteria set at higher standards than even the currently accepted regulatory guidance. An example would be setting ISR acceptance at ³ 80 %, where the current accepted rate is ³67%
A quality bioanalytical lab team is also committed to continuous improvement, adhering to GLP certification standards, and working directly on feedback from clients during audits to refine processes and enhance the level of service delivery, understanding that the work performed is a partnership aimed to establish and promote a long-term relationship. Bioanalytical labs that achieve low error rates and demonstrate high reproducibility of results (ISR) are not only appreciated by clients, but in turn this can be translated into cost savings in pricing models and timelines and consistently delivered.
Repeat Business
When a quality lab has a reputation for consistently providing high-quality, accurate and reliable data to clients, the clients are more likely to return for future work as they trust the capabilities of the team. Due to the focus on high-quality, with low failure rates requiring repeat work, a bioanalytical lab is recognized as being highly organized and efficient, and they have reliability in the on-time delivery of results. This expedites decision-making processes for clients and facilitates them returning for future studies.
Operation Efficiency and Reduced Cost-of-Services
A bioanalytical lab compliant with OECD GLP certification standards, and built on quality, can lead to reduced costs of services through built-in measures that improve operational efficiency and effectiveness. Rigorous quality measures are developed to minimize errors, ensuring results are reliable and accurate. This significantly reduces the need for rework, repeated experiments, and event investigation, which can be costly and impact timelines. “Doing it right the first time”, allows for cost-of-service savings to be considered in the pricing structure offered to the client. Streamlined workflows designed to maximize productivity also reduces the time required to complete projects, minimizing the need for overtime work. Minimizing waste through resource optimization using lean management principles in the selection and use of appropriate extraction supplies and equipment use also helps to reduce the cost-of-services.
Job Satisfaction/Staff Retention
A bioanalytical lab built on a foundation of strong quality can enhance job satisfaction for its employees and in turn help with staff retention. Such labs are committed to providing professional growth of their staff. Employees feel they have opportunities to advance their careers within the organization, giving them an incentive to stay with the company. High-quality work is also typically recognized, respected, and appreciated by management, clients and regulators. Having your contribution recognized “feels good”. Feeling valued and appreciated is a powerful motivator to staff. Working in an environment with a strong quality framework and efficient and well-organized work processes also contributes to a low-stress and more positive work environment with a good work-life balance.
Bioanalytical labs that consistently produce high-quality results also have a stable client base. A stable and secure work environment is conducive to job satisfaction and retention. Quality focused bioanalytical labs often foster a culture of teamwork and collaboration. In a positive and collaborative work environment, employees work together to maintain the high standards and are committed to facing and overcoming challenges together. High quality staff are often led by experienced and competent leaders who provide clear direction, creating a sense of stability and a positive work-culture.
Positive Regulatory History
A bioanalytical lab that is not only committed to the strict adherence to regulatory guidance, but one that consistently exceed these standards gains regulatory trust and reduced concerns during regulatory audits. In fact, this promotes a collaborative inspection process and ultimately a positive outcome. This can be reflected in the positive regulatory inspection history.
Bioanalytical teams with strong Quality Control (QC) and Quality Assurance (QA) procedures take pride in their documentation practices. These provide regulators with total transparency and confidence that the lab is continuously and rigorously monitored. Demonstrating a proactive approach to corrective and preventative actions (CAPA), which are transparent during inspections, is also welcomed by regulators. In maintaining a strong track record, the bioanalytical lab team becomes known to regulators. Regulators develop trust in not only laboratory management’s philosophy but the teams’ capabilities and commitment to data quality and integrity.
Working in a pharmaceutical research environment, unexpected or unforeseen events can and do occur. Such events should be approached through rigorous SOP-driven processes. Quality labs use detailed experimental designs, focused on interpretation of data, planned investigative procedures, corrective and preventive actions, and an impact assessment on final reported data. Inclusion of such detailed investigations in submitted bioanalytical reports, have also become a regulatory expectation in the activities of bioanalytical labs. This demonstrates to regulators that the credibility and integrity of the submitted data are not questionable.
Regulatory History of BioPharma’s Bioanalytical Laboratoy
| Inspection Agency | Year |
| UK MHRA | 2013 |
| OECD GLP certification, Standards Council of Canada | 2015 |
| FDA | 2015 |
| ANVISA Certification | 2015 |
| UK MHRA | 2016 |
| French National Agency for Medicines and Health Products Safety (ANSM) | 2017 |
| ANVISA Certification | 2017 |
| OECD GLP certification, Standards Council of Canada | 2017 |
| FDA | 2017 |
| Danish Medicines Agency (DKMA) | 2017 |
| WHO | 2018 |
| FDA | 2019 |
| ANVISA Certification | 2019 |
| OECD GLP certification, Standards Council of Canada | 2019 |
| UK MHRA | 2019 |
| OECD GLP certification, Standards Council of Canada | 2021 |
| ANVISA Certification | 2021 |
| FDA | 2022 |
| Austrian Agency for Health and Food Safety (AGES) | 2022 |
| Health Canada | 2022 |
| Danish Medicines Agency (DKMA) | 2022 |
| Dutch Medicines Evaluation Board (CBG) | 2022 |
| OECD GLP certification, Standards Council of Canada | 2023 |
| WHO | 2023 |
Table as of January 2024.
Why Choose BioPharma Services
The bioanalytical lab of BioPharma Services Inc. consistently commits to uphold their proven track record for scientific excellence, perseverance, and unwavering commitment to provide services of the highest quality. Our efficient laboratory workflows are designed to reduce unnecessary repeat work/additional work, and they do just that. Our track record for quality is reflected within the ruggedness of our ~ 300 validated methods and their excellent performance when applied to sample analysis studies. This drive for excellence is also reflected in successful regulatory audits and due to our expert bioanalytical lab we have become known as industry standard setters in bioanalysis.



