Bioanalytical Method Development: High PH Mobile Phase

One of the unique characteristics of human beings is our urge to go beyond the limits set for us by our environment. Science is one of the tools that humans use to achieve this goal. In 1968, Malcolm Dole reported unusual ion current traces after atmospheric pressure nebulization of an electrically charged solution of polystyrene.
In his book, Patrick Arpino, describes Malcolm Dole’s discovery as humans breaking the ”volatility barrier,” just as Captain Charles broke the ”sound barrier” in 1947 by flying his newly designed Bell X-1 aircraft faster than the speed of sound. The predictions that the ”sound barrier.” or ”volatility barriers” could not be broken were wrong. After another 25 years, based on Malcolm Dole’s breakthrough, completely new analytical instruments were designed. These instruments are generically termed liquid chromatography mass spectrometers (LC-MS). Today, LC-MS is considered as a powerful analytical tool and has been widely used in pharmaceuticals, biotechnology, environmental monitoring, food processing, and other fields.
Utility of LC-MS in Bioanalysis
LC-MS is a chemistry technique used in bioanalysis that combines the physical separation capabilities of liquid chromatography (or HPLC) with the mass analysis capabilities of mass spectrometry. It is very useful for analyzing small molecules and offers higher sensitivity and selectivity in the trace analysis of multicomponent containing substances.
LC-MS is used in the pharmaceutical industry to provide quantitative measurements of an active drug and its metabolites to generate accurate pharmacokinetic, toxicokinetic and bioequivalence data, which are fundamental to the clinical drug development process. The technique is also used for the analysis of biological matrices in a number of other fields (e.g., sports doping, forensic toxicology).
Why pH is Important in Liquid Chromatography
The International Union of Pure and Applied Chemistry (IUPAC) defines chromatography as follows: “Chromatography is a physical method of separation in which the components to be separated are distributed between two phases, one of which is stationary (the stationary phase), while the other (the mobile phase) moves in a definite direction.” In HPLC, the mobile phase is a liquid delivered under high pressure (up to 400 bar (4 × 107 Pa)) to ensure a constant flow rate, and thus reproducible chromatography.
The stationary phase is packed into a column and is capable of withstanding the high pressures which are necessary. The pH and ionic strength of the aqueous portion of the mobile phase can affect chromatography in a number of ways. Depending on the compound being analyzed, pH can impact selectivity, peak shape, and retention.
The Benefit of High pH Mobile Phase
Weakly basic compounds account for a large proportion of pharmacologically active compounds. Due to these compounds’ basicity, they generally bear a positive charge in aqueous solutions of acidic or neutral pH. This could lead to poor chromatographic performances, overloading phenomenon, and poor retention characteristics[6]. LC-MS/MS operated under reversed-phase chromatography and high pH mobile phases has shown marked advantages for the analysis of highly polar basic drugs. Under high pH mobile phase conditions, basic molecules become deprotonated and therefore less polar. The stronger retention allows the basic compounds to be eluted from the column with a mobile phase rich in organic solvent, and generally leads to higher sensitivity with an electrospray ionization (ESI) source.
Moreover, the higher retention of the highly polar basic drugs compared to the solvent front avoids drastic ionization suppression from endogenous constituents in biological matrices such as plasma, whole blood and urine. Due to the change in ionization state of basic solutes, the selectivity of a HPLC separation can also be significantly improved under high pH mobile phase conditions.
Case Study: The Bioanalytical Method for Ivabradine and its Metabolite in Human Plasma
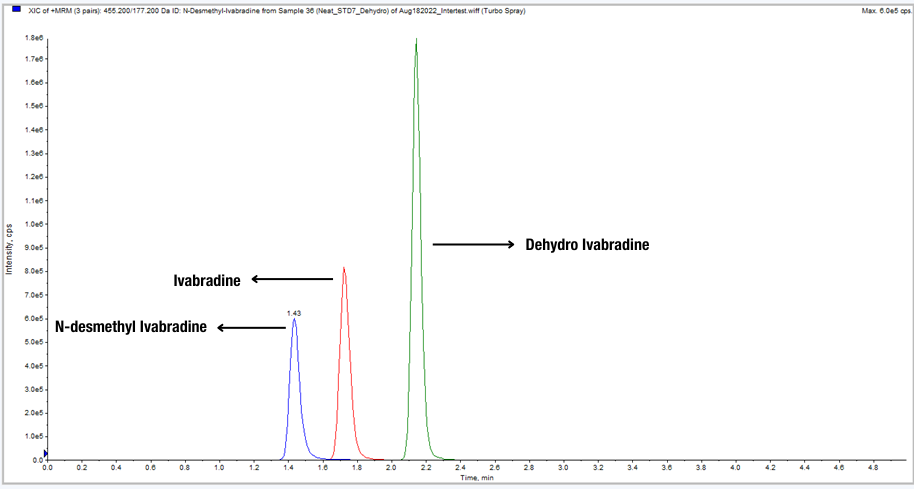
A sensitive LC–MS/MS method for simultaneous quantitation of Ivabradine and its active Metabolite N-Desmethyl Ivabradine was developed. The linear dynamic range of the method is from 0.10 to 100.0 ng/mL for Ivabradine and 0.01 to 10 mg/mL for N-Desmethyl Ivabradine. The inactive metabolite dehydro Ivabradine was also tested as interference during the method validation at 2.50 ng/mL in plasma sample. Dehydroivabradine differs from Ivabradine by only 1 atomic mass unit (amu), and therefore cannot be distinguished from ivabradine by mass spectrometric alone.
The most challenging aspect of this method is the LC separation of three compounds. Without separation, Dehydro Ivabradine could affect Ivabradine’s peak shape and hence the quantitative accuracy of the method.
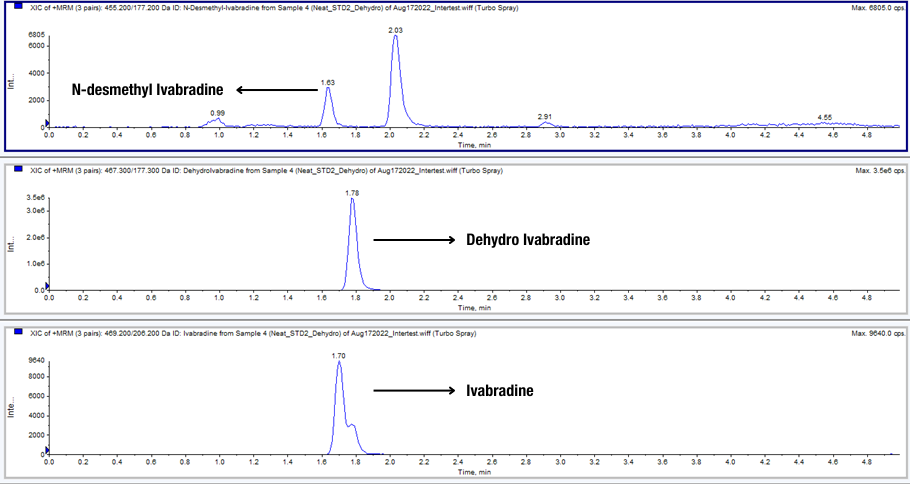
After carefully optimizing the mobile phase and columns, the three components were successfully separated on a Waters X-Bridge® column using gradient elution and a high pH mobile phase. The method was fully validated and applied in bio-studies.
Why Choose BioPharma Services?
At BioPharma Services, we perform LC-MS/MS analysis using AB Sciex triple quadrupole mass spectrometers equipped with HPLC systems. Our chromatographic separation capabilities include reverse-phase, ion-pair-, and HILIC separation techniques for a wide range of small molecule pharmaceuticals in various matrices such as whole blood, plasma, urine and cerebrospinal fluid.
All methods are developed to include the assessment of the impact of major metabolites that would be present in clinical samples. During bioanalytical method development, high pH mobile phase is a powerful technique in the “chromatography toolbox” to control analyte retention, peak shape and selectivity to allow the development of accurate, precise and reliable assays for application in clinical research and non-clinical (GLP) studies.
Written by: Hongzhi liu and Dr. Nicola (Nicki) Hughes
BioPharma Services, Inc., a Think Research Corporation and clinical trial services company, is a full-service Contract Clinical Research Organization (CRO) based in Toronto, Canada, specializing in Phase 1 clinical trials 1/2a and Bioequivalence clinical trials for international pharmaceutical companies worldwide. BioPharma has clinical facilities both in the USA and Canada with access to healthy volunteers and special populations.



