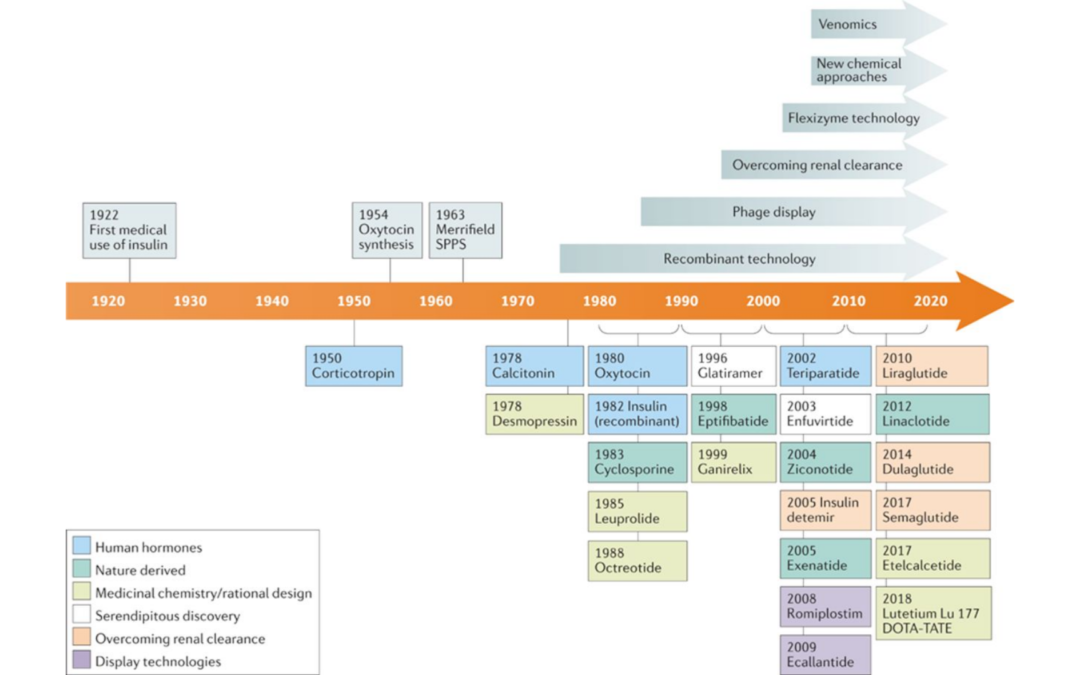

Bioanalysis Considerations for Endogenous Substance Drug Products
Bioanalysis Considerations for Endogenous Substance Drug Products Wait! We think you’ll find the following related article of similar interest. BIOANALYTICAL METHOD VALIDATION FOCUS ON SAMPLE STABILITY. To Read, Click Here. The term endogenous is the opposite of...
Bioanalytical Method Validation Focus on Sample Stability
Bioanalytical Method Validation Focus on Sample Stability Bioanalytical method validation is performed to demonstrate that a given method is suitable for its intended use, i.e., in the quantitative analysis of drugs, metabolites and biomarkers in biological matrix...
Oncology Drug Development Formulation in Clinical Research Trials
Oncology Drug Development Formulation in Clinical Research Trials BPSI’s success story using normal healthy volunteers for Cost-efficient clinical development of a generic or improved formulation of oncology drug(s) Introduction Growing concerns about the high number...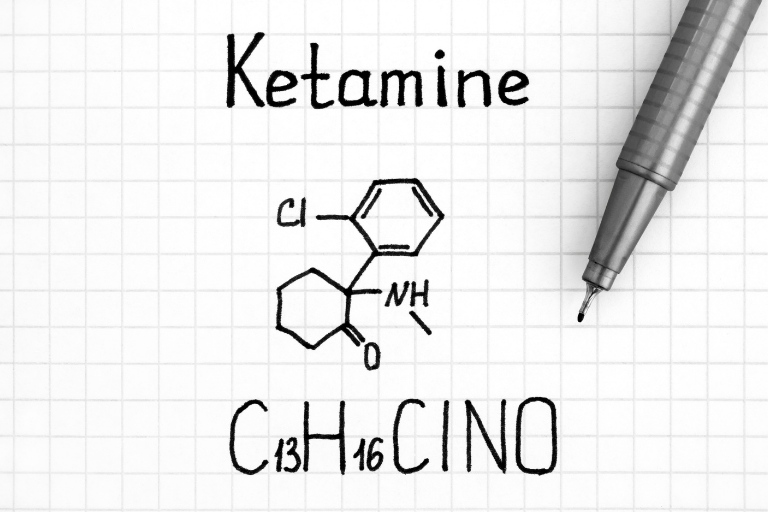
Clinical Research Spotlight: Ketamine, Norketamine and Hydroxynorketamine
Clinical Research Spotlight: Ketamine, Norketamine and Hydroxynorketamine Ketamine is a precipitation drug that was developed in the 1960s and approved as an anesthetic. Ketamine has been used on the battlefield to treat injured soldiers in the Vietnam War, in...

Recent Comments