NDA Enabled Phase 1 Clinical Trials: Unveiling the First Step

The journey of a new drug from concept to market is a complex and highly regulated process. At its core lies the New Drug Application (NDA) submission – a critical milestone that paves the way for new medications to reach patients in need. But how do drugs reach this stage of approval? The answer often begins with Phase 1 clinical trials.
In this blog post, we will explore the significance of Phase 1 clinical trials and the various types of trials that are conducted within this phase, setting the stage for the successful submission of an New Drug Application.
Phase 1 Clinical Trials – The Foundation of New Drug Application:
NDA Phase 1 clinical trials serve as the bedrock upon which the entire drug development process is built. These trials are the first step in testing an experimental drug in humans, and they are designed to address several key objectives.
Key Components of Successful New Drug Application:
New drug safety: The primary goal of NDA Phase 1 trials is to assess the safety of the experimental drug in humans. Before administering the drug to a larger patient population, it’s essential to ensure it doesn’t cause harm. These trials typically involve a small group of healthy volunteers who are closely monitored for any adverse effects.
Dose finding: Determining the optimal dosage of the investigational drug is another vital aspect of Phase 1 trials. Researchers aim to strike a balance between effectiveness and safety, identifying the right dosage that maximizes the therapeutic benefit while minimizing potential side effects.
Pharmacokinetics and Pharmacodynamics: These studies delve into how the body interacts with the drug. Pharmacokinetics examines how the drug is absorbed, distributed, metabolized, and eliminated, while pharmacodynamics explores the drug’s effects on the body.
Initial Efficacy: While the primary focus of NDA Phase 1 trials is safety, in some cases, these studies can also provide preliminary data on the drug’s effectiveness. Proof-of-Concept (PoC) trials, a specific type of Phase 1 study, may offer insights into the drug’s therapeutic potential.
Types of Phase 1 Clinical Trials:
Within the Phase 1 NDA stages, there are several types of clinical trials, each with a specific focus and set of objectives.
The Phase I trials are generally conducted in the following sequence: single ascending dose, multiple ascending dose, examination of preliminary effect of food on exposure, and any potential drug-drug interaction, with assessments to determine the effect of gender, age, bioavailability and bioequivalence performed as required. Other trials such as TQT (Through QT), HAL/HAP (human abuse liability/potential), potential alcohol interaction etc. may be required depends on the particular compound has been developed.
Single ascending dose (SAD) & multiple ascending dose (MAD) are integral part of the Phase I trials. SAD and MAD help in determining how the drug is processed within the body and how well it is tolerated by the body, and these are the
Single Ascending Dose (SAD):
A small group of subjects/healthy volunteers receive a single dose of study drug while being observed and tested for a period of time to confirm safety and characterize the PK of the study drug, where safety and PK assessments are done for a predefined time.
The SAD study steps: Initially a small number of healthy volunteers (usually n~6 or 8) are dosed with one particular dose (x1). If there is no adverse event and the pharmacokinetic (PK) data are roughly in line with predicted safe values: the dose (x2) is escalated, either within the same group or a further group of healthy subjects who are then given the higher dose (x2). Dose escalation continues until the maximum dose was attained as per protocol unless a predefined maximum exposure is reached or unacceptable side effects were apparent.
Dose escalation proceeds according to the protocol assuming strict safety and PK criteria are met. If unacceptable toxicity is observed in any of the three participants, an additional number of volunteers, are treated at the same dose. This is continued until pre-calculated pharmacokinetic safety levels are reached, or intolerable side effects start showing up (at which point the drug is said to have reached the maximum tolerated dose [MTD]). If an additional unacceptable toxicity is observed, then the dose escalation is terminated and that the previous dose, is considered as the maximum tolerated dose. Variations to this design do exist, but most are similar ones. SAD studies mostly include sequential groups in a parallel design for maximum exposure or have a crossover design to provide more information on dose linearity. Studies are usually placebo controlled to determine whether effects observed are due to the study drug or environmental conditions.
Multiple Ascending Dose (MAD):
Withing the NDA Phase 1 trials, Multiple ascending dose studies investigate the pharmacokinetics and pharmacodynamics (PK and PD) of multiple doses of the drug, looking at safety and tolerability. (in nutshell Mad studies check for safety/tolerability and PK/PD).
The MAD study steps: The dose levels and dosing intervals (i.e., time between consecutive doses) are selected based on the prediction from single dose study data (safe dose/MTD). The dose is subsequently escalated for further groups, up to a predetermined level.
Dose levels and dosing frequency are chosen in order to achieve therapeutic drug levels within human systemic (blood) circulation which is maintained at steady state for several days to allow appropriate safety parameters to be monitored. This involves enrolment of healthy volunteers or patients depending on the results from the SAD studies or can be an extension of SAD study as well.
A group of healthy volunteer/patients receive multiple low doses of the drug, while samples (of blood and other fluids) are collected at various time points and analyzed to acquire information on how the drug is processed within the body. Blood samples are collected and analyzed from study participants to allow the determination of PK profiles and a better understanding of how the drug is processed by the body; with multiple dosing, a key part of the PK analysis of MAD study is to identify if accumulation of the drug occurs. To reduce the costs of drug development and time to market, we can have SAD and MAD in the same study design. Sometimes it also involves assessment of food effect, and/or sex.
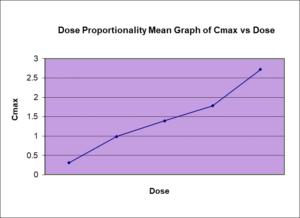
Both SAD and MAD studies are used to gain the knowledge of the maximum tolerated human dose and can also assess the dose proportionality with the doses given.
Food effect studies:
Evaluation of the effect of food on the pharmacokinetics of oral drugs is critical to drug development, as food can mitigate or exacerbate toxicities and alter systemic exposure. These NDA Phase 1 trials investigate whether the drug’s absorption and effectiveness are influenced by food. Food-effect study usually a crossover design with subjects number in the range of 12 to 40 or more depends on the drug’s pharmacokinetic property. Starting food-effect studies as early as possible is key to inform dosing in pivotal trials.
Age/gender studies:
These trials investigate whether the drug’s absorption and effectiveness are influenced by age and sex. Understanding this can be crucial in determining how patients should take the medication and should investigate as early as possible.
Bioavailability and Bioequivalence (BA/BE) studies:
Bioavailability is referred to as the extent and rate to which the active drug ingredient or active moiety from the drug product is absorbed and becomes available at the site of drug action. The relative bioavailability in terms of the rate and extent of drug absorption is considered predictive of clinical outcomes. Bioavailability studies compare the investigational drug to a reference formulation, ensuring they are similar in terms of efficacy and safety. Bioequivalence studies assess generic drugs compared to their brand-name counterparts. BA/BE studies are critical during the drug development process to facilitate the understanding of the
Drug-drug Interaction (DDI) studies:
These NDA Phase 1 trials assess how the investigational drug interacts with other medications that patients may be taking. Ensuring there are no harmful interactions is essential for patient safety.
In general, there are 2 drugs involved in a drug-drug interaction study, and they should always interact in the same way. One drug is considered the probe substrate (inhibitor or inducer), and the other is the interacting drug. The probe substrate is metabolized or transported by the biological enzyme being studied (e.g. cytochrome P450 3A4). The interacting drug affects the biological enzyme being studied by either inhibition or stimulation.
To explain a specific example, let’s assume one evaluates the potential for a candidate drug to inhibit cytochrome P450 3A4. The candidate drug will be the “interacting drug” and selected probe substrate will be sensitive to changes in CYP3A4 activity (e.g. midazolam).
The DDI study is usually the crossover design with the administration of two separate treatments to each subject. Because the biological enzyme that is considered to be free from influence of the study subject, blinding is not always necessary in DDI studies. Therefore in an open label study, a fixed sequence design is the easiest to conduct and can be used. The basic study involves evaluating the clearance of the probe substrate under two conditions:
| Condition | Treatment | Common term |
| 1 | Probe substrate alone | Reference |
| 2 | Probe substrate + interacting drug | Test |
In crossover DDI design, a subject receives treatment 1 (probe substrate alone) in the first period, followed by an appropriate washout. Then the same subject receives treatment 2 (probe substrate + interacting drug) in the second period. By comparing PK results (AUC) from period 2 to period 1, one can determine the effect of the interacting drug. We will calculate the relative exposure between the Test and Reference using a ratio of AUC (Test/Reference). If the ratio is close to 100%, then the interacting drug did not affect the clearance of the probe substrate. If the ratio is >100%, then the interacting drug inhibited the clearance of the probe substrate. And a ratio <100% suggests that the interacting drug stimulates the clearance of the probe substrate.
Additionally, multiple doses of the interacting drug to achieve maximal inhibition or stimulation is necessary, and in general the interacting drug is dosed to steady-state levels.
Through QT studies:
A thorough QT (TQT) study is in vivo studies for new drugs and should take place prior to Phase 2/3 clinical trials normally has two hypotheses. The primary hypotheses is to assess if the test treatment will not prolong QT interval. The second hypotheses is to determine the assay sensitivity of the positive control treatment in the study population. The QT studies are typically single dose crossover studies with healthy subjects, evaluating the therapeutic and supratherapeutic doses of the drug versus a positive control and negative control (placebo).
Human abuse potential/liability studies:
Human abuse potential (HAP) studies, also known as human abuse liability testing, have gained relevance as the FDA becomes increasingly interested in knowing whether or not new drugs could be misused for recreational purposes. One is a more traditional study that is required for new chemical entities. If this new drug is known to target the central nervous system (CNS), then the FDA wants to know if it has the potential to attract the recreational drug community because of its effects.
Human abuse potential (HAP) clinical trials are used by the FDA to formulate scheduling recommendations for drug products containing a controlled substance, and are also the Category 3 studies used to assess abuse deterrence. HAP studies are conducted in recreational drug users who have experience with the route of abuse being studied. The preferred study design is a randomized, double-blind, placebo- and active-controlled crossover study in which the abuse-deterrent formulation is compared to a positive control and the positive control is compared to placebo in order to validate the study.
The main method used to evaluate the subjective pharmacodynamic effects of the study drugs are standardized instruments called visual analogue scales (VASs). The VAS for drug liking is the primary measure, since it correlates most directly with the potential for abuse. Other secondary endpoints of interest include the likelihood of taking the drug again and the high experienced by the subjects. If the study drugs are snorted, then additional measures relevant to the intranasal route of administration are included (eg, intranasal irritation).
The general design of HAL/P studies are randomized crossover designs with the placebo and positive control arms. The overall assessment of abuse potential and deterrence is based on the pattern of findings across all subjective measures, including both positive and negative effects (eg, nausea).
Special population (hepatic impairment -HI or renal impairment – RI) studies:
Most drugs are likely to be administered to RI or HI patients during the treatment and should be investigated in such populations.
Drugs are normally cleared from the human body by a number of routes, each of which can be characterized by a clearance value. The sum of these clearances (renal, hepatic, etc.) is the total or body clearance which is inversely proportional to the plasma concentration produced by a given drug dosage regimen. The quantitative contribution of each route of elimination to the metabolic fate of a drug is proportional to the clearance value of that route relative to the body clearance. The reduction in renal clearance of a drug caused by renal disease is proportional to the reduction in the renal clearance of creatinine.
Liver is the primary site for clearance of xenobiotics utilizing mainly cytochrome P450 enzyme pathways, glucuronidation, and biliary excretion. It’s understandable that HI can alter drug absorption and disposition (i.e., pharmacokinetics [PK]) and consequently their efficacy and safety or pharmacodynamics (PD). In general, regulatory agencies recommend conducting PK studies in subjects with HI when hepatic metabolism/excretion accounts for more than 20% of drug elimination or if the drug has a narrow therapeutic range. There is current available FDA guidance for study design and conducted for HI populations. The trial will be conducted in a randomized, double-blind design. Participants will be divided into at least three groups: a control group with normal renal function, groups with renal impairment with minimum of mild or moderate hepatic functions.
The reduction in renal clearance of a drug caused by renal disease is proportional to the reduction in the renal clearance of creatinine. The trial will be conducted in a randomized, double-blind design. Participants will be divided into at least three groups: a control group with normal renal function, groups with renal impairment with minimum of mild or moderate renal impairment based on their creatine clearance as per regulatory guidance. The participants will be administered the drug at a fixed dose, and blood samples will be collected at various time points to measure the concentration of the drug in the bloodstream. The pharmacokinetic parameters that will be evaluated in the trial include the maximum concentration (Cmax), the time to reach maximum concentration (Tmax), and the area under the concentration-time curve (AUC). These parameters will be used to determine how the drug is absorbed, distributed, metabolized, and eliminated in the body, and to compare the pharmacokinetics of the drug in individuals with normal renal function to those with impairment.
Overall, while Phase 1 clinical trials are crucial, they are challenges and risks involved in such trials. The potential for adverse events, even with rigorous safety protocols in place, means that close monitoring and risk mitigation strategies are necessary.
The Role of Phase clinical trials of New Drug Application (NDA) submission:
Phase 1 trials are just the beginning of a drug’s journey. They provide a foundation upon which subsequent phases (Phase 2 and Phase 3) build. Accumulating substantial safety and efficacy data is essential before proceeding to the New Drug Application submission stage.
NDA Phase 1 clinical trials are the cornerstone of the drug development process, serving as the critical bridge to New Drug Application (NDA) submission and eventual market approval. Certain Phase 1 trials are taken longer time to plan and conduct such as special population studies, TQT trials and HAP/HAL clinical trial requires special population, and therefore early engagement of such trials will help to reduce the overall length of the New Drug Application program. food, age, gender effect may provide the good understanding for the planning of the Phase 2 or 3 clinical trial so early assessment will be beneficial. Understanding the different types of Phase 1 trials and their significance is vital for drug development.