Bioanalytical Method Development The Misnomer that Urine Bioanalysis is Simple
Diseases have been with mankind since the beginning of time, and medicines have helped countless people to overcome them. Being cured of diseases with the help of drugs is almost everyone’s wish. The development of modern science and technology has brought with it a qualitative leap to human society, and the speed of drug discovery has been greatly accelerated.
Drug evaluation methods have also been continuously improved, from the early evaluation of the efficacy and side effects of drugs in animal models to the confirmation of the safety and reliability of drugs in large-scale clinical trials, making drug evaluation more standardized and the results more reliable.
Bioanalysis is the quantitative determination of drugs and their metabolites in biological fluids. Urine bioanalysis is one of the most common bioanalytical methods. Bioanalytical techniques are used very early in the drug development process to provide support to drug discovery programs on the metabolic rate and pharmacokinetics of chemicals in, in vitro cell systems and in animals. The emergence of the field of bioanalysis, as a critical tool, is globally recognized during the preclinical and clinical stages of drug development. Within this context, the role of urine bioanalysis cannot be understated.
Why the Need to Know Drug Concentrations in Human Urine?
There are many factors that affect the efficacy of a drug, one of them being pharmacokinetics, which refers to the process by which a drug enters the body, passes through the body, and is excreted from the body. Pharmacokinetics is the study of how the body interacts with the administered substance throughout the exposure process. This field typically studies four main parameters: absorption, distribution, metabolism, and excretion (ADME). Excretion is the process by which a drug is removed from the body. Assessment of urinary excretion is an important factor in understanding the metabolic pathway of a compound.
For drugs that are excreted from the urine primarily in an unchanged form, bioavailability can be estimated by measuring the total amount of drug excreted after a single dose. Clearance is an important term when studying excretion; it is defined as the ratio of the rate of drug elimination to the plasma drug concentration. According to current pharmaceutical industry practice, if the urinary excretion of a drug candidate and/or its active metabolite exceeds 10% of the administered dose, its concentration in the urine needs to be measured.
The Challenges in Bioanalysis Urine Sample.
Urine consists of greater than 95% water, so at 1st glance, urine may be considered a simple matrix. However, in addition to water, it contains various byproducts of metabolism, and includes approximately 500 different constituents including urea, chloride, sodium, potassium, and creatinine as well as other organic and inorganic salts and byproducts of metabolism of endogenous compounds. The pH of urine can also vary in a normal range of 5.7–7.0. Urine salt concentrations can also vary from the subject and the degree of hydration. In contract to other biological matrices (whole blood, Plasma Serum etc.) in normal healthy individuals, urine does not normally contain protein and lipids.
In quantitative urine bioanalysis, the lack of protein and lipids can be associated with the issue of nonspecific binding or container surface adsorption of drug molecules, especially for lipophilic and highly protein bound drugs that actively seek surfaces for attachment to reduce lipophilicity. Nonspecific binding can result in nonlinear response of spiked calibration standard curves; poor reproducibility of the assay, especially at the lower concentration level and the large bias compared to nominal values; poor extraction recovery of analyte after one or more freeze-thaw cycles.
Variable pH or ionic strength of urine can lead to inconsistent extraction recoveries, matrix effects and nonreproducible analyte LC-MS/MS response between runs. In fact, compared to other matrices (whole blood, plasma, serum etc.), urine is a very heterogeneous and therefore a challenging matrix.
Here we describe 2 case studies, the 1st being a very simple bioanalytical method and the second showing an example of the special handling requirements that might be required/considered for urine bioanalysis.
Case study 1: Development and validation of a LC-MS/MS method for the determination of Vancomycin in human urine.
Vancomycin is an antimicrobial agent for which therapeutic drug monitoring is recommended to maintain efficacy and safety of therapy. After intravenous administration of vancomycin, almost all of it is rapidly excreted in the urine in the form of an unaltered drug. Therefore, there is a strong demand for vancomycin urinalysis testing and the need to obtain the necessary information to guide vancomycin dosing regimens.
Vancomycin has a molecular weight of 1449.27 grams/mol and is protonated to form a doubly charged protonated molecule when analysed by LC-MS/MS in positive ion mode. For urine bioanalysis, an analog internal standard was used since no stable labeled internal standard was available. The assay utilizes a protein precipitation extraction procedure requiring 50 µL of human urine. Samples were separated chromatographically on an ACE C18 analytical column using a gradient elution. The method has been well validated and applied in pharmacokinetic studies.
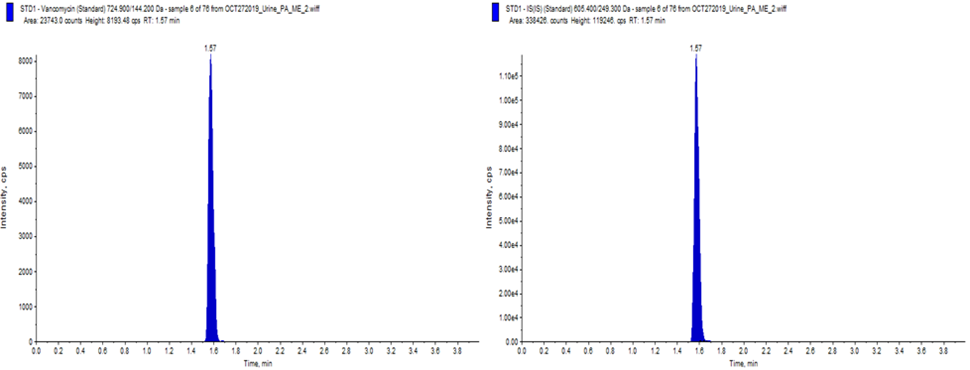
Figure 1: Example chromatogram for Vancomycin in urine (left- Vancomycin) (right- Internal Standard)
Case study 2: Determination of S-Compound in human urine by LC-MS/MS and application to a Phase I study.
S-Compound is a proprietary oncology drug under clinical trials. Firstly, a method was developed for use on plasma samples. The assay utilized a protein precipitation extraction procedure that requires a 50 µL sample to which an analog compound is added as an internal standard. The sample was chromatographically separated on a Phenomenex Kinetex Biphenyl column with a gradient elution. Based on the assay in plasma, a simple extrapolation of the method for application to urine samples was expected.
During the development of the urine bioanalysis method, however, a nonlinear standard curve was observed. Upon investigation, it was found that the non-linear standard curve was caused by non-specific binding of the analyte to the wall of the sample container. To avoid this non-specific binding, blank urine was treated with a 30% commercial bovine serum albumin (BSA) solution to give a final concentration of 0.9% BSA in urine. Under these conditions, the standard curve prepared with the treated blank urine showed a linear relationship between peak area ratio and analyte concentration.
The reason for the non-linearity of the untreated standard curve is that more analyte is lost in samples with low to medium concentrations and relatively less analyte is lost in samples with high concentrations. After preparing a urine standard curve using 0.9% BSA urine, the analytes were not adsorbed to the sample container, making the standard curve linear.
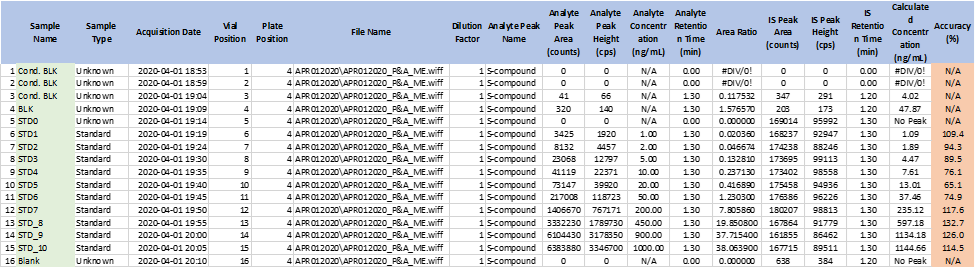
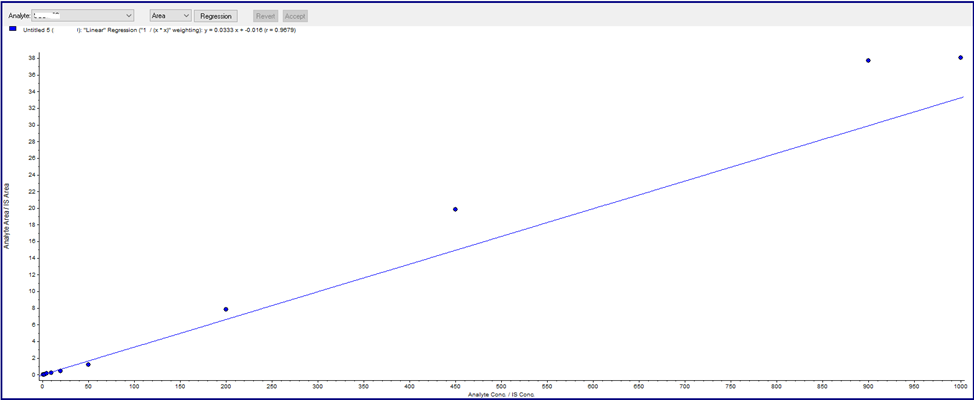
Figure 2: Non-Linear Urine Calibration curve (No BSA)
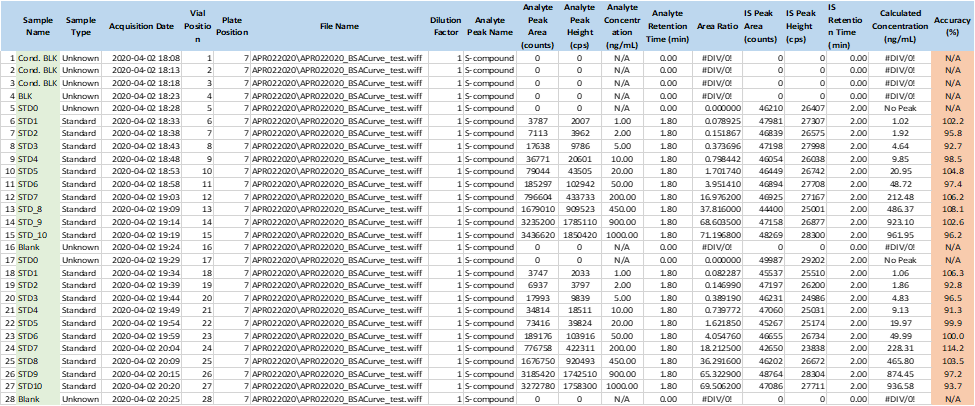
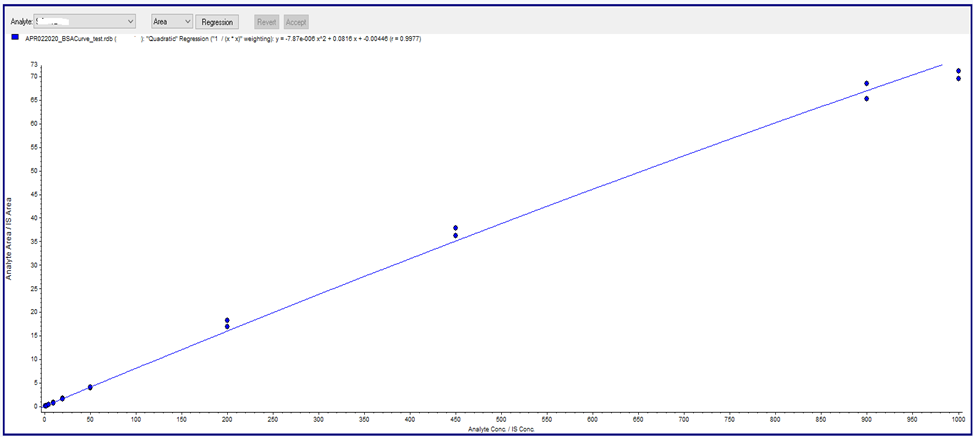
Figure 3: Linear Urine Calibration Curve (with 0.9% BSA)
After this observation, it was determined that all urine sample collection containers must be rinsed with 30% BSA and allowed to air dry when urine samples are collected at the clinical site. To support the clinic’s sample collection procedures, experiments were conducted to evaluate the effect of different concentrations of BSA; Low QC samples (LQCs) were prepared with varying percentages (0.15% to 5%) of BSA and analyzed against a standard curve prepared in urine containing 0.9% BSA. The results showed that small differences in BSA levels in urine samples do not affect the quantification of the samples.
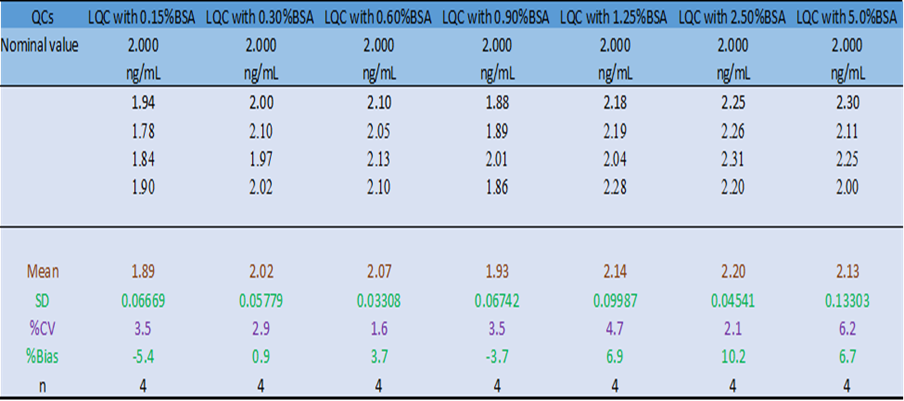
Table 1: The impact of different percentage of BSA in urine on quantitation of S-Compound, (Standard Curve was prepared in 0.9% BSA in Urine)
Why Choose BioPharma Services for Your Next Drug Development Project.
Urine samples have varying pH values, varying salt concentrations (ionic strengths), varying buffering capacities, as well as hundreds of endogenous constituents with similar structure and polarity to the analyte of interest. Due to the high aqueous contact and lack of proteins and lipids, there is a high potential for loss of analytes in urine due to non-specific binding of the analyte to the surface of the container. Clinical sample collection procedures require careful and often special evaluation; urine is NOT a simple matrix.
At BioPharma Services Inc., the R&D team have successfully developed and validated approximately 300 bioanalytical assays in biological matrices, including whole blood, plasma, serum and the urine bioanalysis. These bioanalytical method validations include prodrugs, therapeutic peptides and polar compounds. Based on our extensive experience, we always strive to develop the right method for the right sample matrix. Meeting and exceeding the satisfaction of our customers is our top priority.
Written By: Hongzhi Liu, Principal Research Scientist & Dr. Nicola Hughes, Sr. VP of Bioanalytical Lab Operations.



